Abstract
Understanding spatial distribution and temporal variation in movement patterns of closely related species is relevant for deciphering how resources are selected and whether interactions between species affect resource use patterns. The horizontal space use and vertical space use of two exploited reef fish, Plectropomus leopardus and P. laevis (all adults), were compared at mid-shelf Helix Reef and Lodestone Reef in the Great Barrier Reef over ~3 years using passive acoustic telemetry. Both species were detected throughout the 12-month duration of transmitters (mean detection period: ~270 days) and often made deep movements to ~40 m possibly related to reproductive behaviour (spawning). Differences in space use were apparent between species, with P. laevis consistently using greater area around reefs throughout the year. Overall, depth use patterns were similar between species; however, when daily detections were grouped in 2-h periods, P. laevis remained shallower and had greater variation in depth use compared to P. leopardus. Contrasting patterns of space use between these co-occurring species, in conjunction with known dietary dissimilarities, indicate distinct habitat use and resource preferences that are important for conservation and fisheries management.
Similar content being viewed by others
Introduction
Coral reef fish have important ecological and economic value, but are increasingly at risk of population declines from human (Sadovy 2005; Newton et al. 2007) and environmental (Emslie et al. 2015; Mellin et al. 2016) disturbances. To better understand how species will respond to, or be affected by, direct and indirect stressors such as fishing, habitat degradation, increased water temperature, or altered prey composition, it is essential to know how they obtain resources, meet energetic requirements, and interact within their environment (Botsford et al. 1997; Roessig et al. 2004). Studying movement and space use patterns is a fundamental approach to characterise how reef fish access resources, interpret behavioural activities, and predict ecological consequences (Hussey et al. 2015). Three-dimensional space use of aquatic organisms is of growing interest to ecologists because depth use has a significant bearing on estimates of home range, habitat selection, diel behaviour, and predator–prey interactions, among others (Gleiss et al. 2013; Currey et al. 2015a; Espinoza et al. 2015a). Thus, ecological interpretation of animal behaviour is greatly improved by data that incorporate horizontal and vertical aspects of the aquatic environment. Furthermore, by comparing three-dimensional patterns of activity and habitat use of closely related species that overlap in distribution (sympatry), insight into behaviour such as competitive interactions and niche segregation can be explored (Dance and Rooker 2015; Guzzo et al. 2015). Interactions between sympatric species (e.g. for habitat and prey) can affect how biological and energetic requirements are met (Zaret and Rand 1971; Mueller et al. 2016). These interactions can also have far-reaching implications for predators (Braune et al. 2014) and prey (see Estes et al. 2011) ultimately affecting predator–prey relationships and the movement of energy within an ecosystem. Therefore, studying horizontal and vertical behavioural patterns related to space use of sympatric species aids interpretation of possible resource partitioning, as well as identifies species-specific depth/habitat preferences and home range sizes. This is particularly relevant within commercial or recreational fisheries because resource-based ecological information can help form appropriate management strategies (e.g. spatial and temporal fishing closures—Waldie et al. 2016) and plan for anticipated vulnerabilities (e.g. shifting spatial ranges—Hill et al. 2016) at a species level.
Coral trout (or coralgrouper) Plectropomus (Oken 1817) form the basis of commercial, recreational, and artisanal fisheries in the south-western Pacific including Australia, Indonesia, and Fiji, among others (Sadovy de Mitcheson and Colin 2012). The term ‘coral trout’ incorporates several species of primary (P. leopardus, P. laevis, and P. maculatus) and secondary (P. areolatus, P. oligacanthus, and Variola spp.) fishery significance; however, the importance of each species varies at a regional level. In the Queensland (Australia) Coral Reef Fin Fish Fishery (CRFFF), commercial fishers primarily catch ‘coral trout’ <24 m deep (Little et al. 2008) for live transport to Asia (Sadovy de Mitcheson et al. 2013). Plectropomus comprise ~35–55% of all commercial catch in the CRFFF (Mapstone et al. 2004), which is predominantly P. leopardus (>80%, Sadovy de Mitcheson and Colin 2012). Plectropomus leopardus are sedentary opportunistic/generalist ambush predators that associate with reef structure for protection or camouflage, although mid-water feeding also occurs (Goeden 1978; St. John 2001). They rarely make inter-reef movements (Davies 1996; Sumpton et al. 2008) and typically remain in a relatively small area diurnally and seasonally (<0.5 km2, Zeller 1998; Bunt and Kingsford 2014; Matley et al. 2015). Unlike other large epinephelids, spawning activity of P. leopardus appears to be localised to small groups between September and December (Zeller 1998; Tobin et al. 2013; Carter et al. 2014). The prevalence of long-range movements and large spawning aggregations is likely limited but is not well known. Nevertheless, seasonal spawning-related closures in the fishery occur during 5-day new moon periods in October and November. Due to its widespread abundance and value overseas, scientific research, stock assessments, and commercial logbooks have concentrated on P. leopardus or grouped all species together for simplicity despite biological differences. For example, P. laevis, the second most abundant ‘coral trout’ species at mid-shelf and offshore reefs (Ayling and Choat 2008), grows larger (~100 cm max length) and matures earlier (~1 year) than P. leopardus (matures at 2–3 years; ~60 cm max length) (Ferreira 1995; Heupel et al. 2010), yet no prior study has specifically investigated resource use or movement patterns of P. laevis.
Evidence supports the ecological role of ‘coral trout’ as a high-order predator influencing population dynamics of prey species (Graham et al. 2003; Rizzari et al. 2014; Boaden and Kingsford 2015). The degree or strength of this influence is not known, but ‘coral trout’ likely play an integral functional role in coral reef ecosystems (Heithaus et al. 2008). Although P. leopardus stocks appear to be healthy (Leigh et al. 2014), there is growing concern that over-exploitation (Little et al. 2005; McLean et al. 2011), climate change (Johansen et al. 2015), and extreme weather events (Tobin et al. 2010) will adversely affect sustainability. Similarly, P. laevis is currently listed as ‘Vulnerable’ on the IUCN Red List and without sufficient data, effective management strategies are not possible (Heupel et al. 2010). Considering the relative importance of the ‘coral trout’ fishery and concerns about its future, there is a large gap in knowledge relating to interactions between sympatric species and potential ecological and human-associated impacts. The main goal of this study was to determine whether and how space use differs between two co-occurring species of ‘coral trout’—P. leopardus and P. laevis. Specifically, the following questions were asked: (1) are patterns of residency, home range, and depth use similar between species? (2) Do environmental (e.g. season, time of day, tide, and lunar phase) or biological (e.g. fish size and reef) factors influence horizontal or vertical space use of each species? Passive acoustic telemetry (see Hussey et al. 2015 for review) was used to test these questions because it provides long-term and continuous horizontal and vertical space use data. Findings will help determine whether this multi-species fishery requires greater species-specific attention and will provide novel information about how ‘coral trout’ partition resources.
Materials and methods
Study area and acoustic telemetry
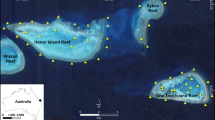
Plectropomus leopardus and P. laevis were tagged with acoustic transmitters during 2013–2014 at Helix and Lodestone Reefs (Table 1). Helix and Lodestone are mid-shelf reefs within the Townsville sector of the Great Barrier Reef Marine Park, Australia. Helix Reef is relatively small compared to Lodestone Reef; at both locations, the reef slope drops from 5 m to 20 m deep within a few hundred metres (Fig. 1).
Map (including contour depth lines) of study sites at Lodestone Reef (left) and Helix Reef (right). Black circles represent the location of moored acoustic receivers and surrounding white contours represent a detection range of 250 m. Bathymetry data were obtained from the Deep Reef Explorer website (http://www.deepreef.org; Beaman 2010)
The acoustic receiver array (2013–2015) consisted of eight VR2W (69 kHz) receivers (Vemco, Halifax, Canada) at each reef (Fig. 1) that were part of a large-scale monitoring study for inter-reef movements of different predatory species. Receivers were deployed along the reef slope and were suspended ~1 m above reef structure using chain and rope, and were 12–19 m deep. Additionally, one receiver was deployed within the reef flat at Helix Reef (~1–3 m deep) for ~1 year to examine frequency of use and cross-reef movements. The average detection range of receivers was ~250 m (Espinoza et al. 2015a), but ranges vary depending on physical and environmental interference around each receiver (Kessel et al. 2014; Huveneers et al. 2016). The detection coverage of acoustic receivers was greater at Helix Reef (>75%) compared to Lodestone Reef (~50%) (see Espinoza et al. 2015a; Fig. 1). Receivers were downloaded every 6 months throughout the study.
Adult P. leopardus (Lodestone Reef—n 32; Helix Reef—n 51) and P. laevis (Lodestone Reef—n 2; Helix Reef—n 10) (Table 1) were captured with a barbless hook (8/0 and 10/0) on line. At the surface, individuals were vented to mitigate barotrauma, tagged externally (dart tag, PDS; Hallprint©), and placed in an anaesthetic bath (Aqui-S® diluted with seawater, 1:10,000). When individuals lost equilibrium, they were moved to fresh seawater for surgery. A V13P (13 × 36 mm) acoustic transmitter (Vemco, Halifax, Canada) was surgically implanted in the body cavity of each individual (see Matley et al. 2015 for detailed surgical methods). Once individuals recovered from the anaesthetic (~10 min), they were released <20 m from their capture site. Each tag randomly emitted a unique identification code every 120 to 200 s with associated depth measurements (±2.5 m manufacturer estimate; <±1.0 m field estimate—see Matley et al. 2015) for an estimated tag life of 352 days.
Analysis
For all analyses, only individuals detected ≥25 times and for ≥15 days were included to avoid individuals with low detections biasing outputs (e.g. fishery captures, mortality events, or moving outside receiver range). If mortality events were apparent during exploratory analysis (e.g. depth sensors followed tidal influences alone), the affected portion of data was removed. Logistic regression (binomial family with logit function) tested whether the inclusion (presence) or removal (absence) of individuals from data analysis was influenced by release distance from a receiver, the size of fish, reef, or release location (i.e. each reef divided into four sections, each containing two receivers: north-east, south-east, north-west, south-west). Validated detections were grouped into 2-h intervals to reduce effects of autocorrelation between consecutive time periods and to estimate individual locations using a position averaging algorithm (see below). Data were verified for normality and heterogeneity using diagnostic plots, and analyses described throughout were considered significant when p ≤ 0.05. All analyses were completed in R version 3.2.4 (R Core Team 2016).
Detection efficiency and presence
To explore diel detection patterns within the arrays, diel receiver efficiency (i.e. proportion of detections each 2 h) was determined at Helix Reef using a range test tag deployed ~125 m away (unobstructed) from a moored receiver for ~3 months. Receiver efficiency was not tested at Lodestone Reef; however, a similar deployment was conducted at John Brewer Reef (~200 m distance between tag and receiver), which is located adjacent to Lodestone Reef and has similar acoustic characteristics (e.g. reef morphology and boating/fishing activity). The mean proportion of P. leopardus and P. laevis detections that occurred during each 2-h interval throughout the study was also calculated for each individual and pooled within-species at Helix Reef and Lodestone Reef.
Monthly measures of residency and roaming were calculated to determine presence within the range of receivers. Proportional residency indices were calculated as the number of days an individual was detected on any receiver each month divided by the total number of days in that month. Roaming was defined as the number of receivers an individual was detected on each month. The first and last month of detections were removed for each individual when measuring residency and roaming unless detections consisted >25 days in that month (at which point residency was calculated accordingly).
Horizontal space use
The occurrence of individual movements between receivers for consecutive 2-h detection periods (generalised linear mixed-effects model (GLMM); binomial distribution) was tested using the glmer function in the lme4 package (Bates et al. 2014). The aim of this logistic regression approach was to test whether movements between receivers were affected by variables such as tidal height (http://www.bom.gov.au), lunar phase (i.e. moon illumination; http://aa.usno.navy.mil/data/docs/MoonFraction.php), fish size, season (summer: December–February; autumn: March–May; winter: June–August; spring: September–November), reef (for P. leopardus only), and time of day. Individual (tag ID) was termed as a random factor. Model averaging (dredge function in MuMln; Barton 2013) was also used to determine which explanatory variable best contributed to the model based on Akaike information criteria corrected for small sample bias (AICc; see Currey et al. 2015b for specific details).
Horizontal kernel utilisation distributions (hKUD) were calculated to compare space use patterns within and between species. Location estimates were based on 2-dimensional positions determined using a 2-h mean position algorithm to derive centres of activity (COAs) (Simpfendorfer et al. 2002). The 2-h period was selected as opposed to the 1-h period used by Simpfendorfer et al. (2002) for sharks because P. leopardus are sedentary in comparison. Therefore, the 2-h period was chosen as a compromise between optimising position estimates (i.e. more time allotted to be detected on multiple receivers) and maximising daily data points (i.e. longer binned periods would reduce temporal resolution of data). Horizontal KUDs representing the core home range (50%) of positions and home range extent (95%) of individuals were calculated using the adehabitatHR package (Calenge 2006). A smoothing parameter (h) of 100 was used to estimate hKUDs based on successive visual trials testing different values (e.g. values that were too high overlapped too much with reef flat areas; values too low underestimated receiver detection ranges). For individuals only detected on one receiver, hKUDs were estimated relative to the average detection range for receivers (250 m—95% hKUD; 125 m—50% hKUD). Horizontal KUDs were calculated at weekly, monthly, and pooled (all detections for each individual) levels. At the monthly level, the first and last month of detections were removed for each individual unless detections consisted >25 days in that month. Species differences were tested (log-transformed) using repeated measures (RM) ANOVAs with individual (tag ID) as a random factor. To test whether the size of individuals or time of year influenced space use estimates, linear mixed-effects (LME) models (nlme in R; Pinheiro et al. 2013) were used for each species (separately) with weekly hKUDs as response variables, fork length (mm), season, and reef (for P. laevis data from Lodestone Reef and Helix Reef were pooled) as explanatory variables, and tag ID as a random factor. The varExp variance structure (nlme; Pinheiro et al. 2013) was used at a monthly level to weight hKUD models to improve homogeneity of variances (Zuur et al. 2009). When categorical factors were significant, contrasts were fitted using the gmodels package (Warnes et al. 2015).
Vertical space use
Transmitter depth data were used to explore vertical space use. A RM ANOVA tested whether overall depth use differed between species throughout the study using the 2-h estimates (log-transformed; tag ID as random effect). Depth values (and their standard deviations) were similarly pooled to compare differences between P. leopardus and P. laevis at each hour (paired t test) and to compare within-individual day/night depth differences (paired t tests) for each species. Linear mixed-effects models were applied to determine whether fish size, season, and/or reef influenced depth use (log-transformed; monthly varID variance structure—Pinheiro et al. 2013) for each species (tag ID as random effect). Similarly, the proportion of depth use >20 m was tested as a response variable (exp(1/2)-transformed) with fish size, season, reef, and location (i.e. north-east, south-east, north-west, south-west) as explanatory variables (tag ID as random effect) to investigate vulnerability of individuals to capture for the live reef food fish trade (~20 m depth cut-off). Proportional estimates were determined at each 2-h COA time period, and grouped for all detections each month and also for each 5-day new moon period (to investigate spawning-related movements) for each species.
Results
Of the 95 individuals tagged with acoustic transmitters, 39 were removed from analyses due to low numbers of detections (Table 1). Of these, one was reported caught by a commercial fisher at Lodestone Reef, and another appeared to have been eaten based on its depth profile. Another three individuals were not incorporated in analyses because they were tagged at and mainly detected on the reef flat receiver (>75% of detections), which was only present for part of the study. No other individuals were detected on the lagoon receiver indicating movements across the reef likely did not occur. On average, individuals were tagged ~110 m (range 0–650 m) from a receiver. None of the factors (i.e. release distance, fish size, reef, and location) significantly explained whether P. leopardus with low detection data were included in analyses or not. Plectropomus laevis was not included in this analysis because of small sample size and high detections at both reefs (e.g. 10/12 individuals had sufficient data). Detection efficiencies calculated from stationary transmitters at Helix Reef and John Brewer Reef showed that receiver detection capability was highest during the day, and more pronounced at Helix Reef (Fig. 2a). Receiver detections from both species were also more common during the day (~7:00–17:00; Fig. 2b).
Were patterns of residency, home range, and depth use similar between species?
Both species had relatively high residency indices typically remaining within the receiver array >50% of days at Lodestone Reef (P. leopardus) and >70% of days at Helix Reef (for both species) each month (Fig. 3a). The mean number of receivers with detections (i.e. a proxy to roaming area or extent) per individual was higher for P. laevis (~4 receivers) compared to P. leopardus (~1–2 receivers) throughout monthly detection periods (Fig. 3b).
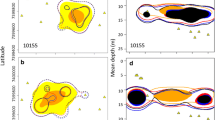
Comparisons of mean hKUDs between species at weekly, monthly, and pooled levels showed that P. laevis used more horizontal area than P. leopardus (p < 0.01 for all comparisons; Fig. 4). There was a gradual increase in the size of 95% hKUDs as temporal resolution decreased, particularly for P. laevis; 50% hKUDs remained constant (Fig. 4).
Based on all 2-h COA estimates, depth use did not differ between P. leopardus and P. laevis (RM ANOVA: F 1,41865 = 2.14, p = 0.15); individuals from both species were primarily positioned between 13 and 18 m throughout the year (Fig. 5a, b). When comparing mean hourly depth use between species, P. laevis moved shallower than P. leopardus (t test: t 11 = −7.21, p < 0.01; Fig. 6a) and had greater variation in depth use (t test: t 11 = 3.61, p < 0.01; Fig. 6b) throughout each day.
Significant seasonal effects on depth from linear mixed effects models for P. laevis (a F 3,8186 = 71.85, p < 0.001) and P. leopardus (b F 3,33670 = 159.27, p < 0.001) and size effects for P. laevis 95% hKUDs (c F 1,221 = 5.64, p = 0.049). Symbols below each plot a, b represent statistically different categories based on contrasts following the mixed-effects models
Did environmental or biological factors influence horizontal or vertical space use?
Time of day and season were the most important variables influencing movements between receivers for P. laevis (Table 2). Specifically, movements between receivers were more common during the day (Fig. 7a) and more frequent during spring and summer (Fig. 7b). Due to the highly resident nature of P. leopardus (i.e. on 96% of consecutive 2-h periods no movements occurred), between-receiver movements were not analysed. The explanatory variables season, fork length, and reef location did not influence home range estimates for either species (p > 0.05), except weekly 95% hKUDs of P. laevis were positively related to fork length (LME: F 1,221 = 5.64, p = 0.05; Fig. 5c).
Plectropomus leopardus were detected deeper at night compared to the day (t test: t 27 = −2.95, p < 0.01; Fig. 6a), while variation in P. laevis depth use was greater during the day (t test: t 5 = 2.61, p = 0.05; Fig. 6b). Season was a significant factor affecting depth use for both species independently: P. laevis tended to be deeper during spring (September–November; Fig. 5a) and P. leopardus moved deeper in spring and summer (September–February; Fig. 5b). Similarly, investigation of the effect of fish size, season, reef, and location on the proportion of detections >20 m identified season as significant for P. laevis (LME: F 3,57 = 4.61, p < 0.01; proportion of deep movements greatest in winter and spring: June-November) and P. leopardus (LME: F3,270 = 8.80, p < 0.01; proportion of shallow movements greatest in autumn: March-May) (Fig. 8). Only reef location (LME: F 3,267 = 5.26, p < 0.01) influenced the proportion of detections >20 m for P. leopardus when 5-day new moon periods were analysed. Specifically, deeper detections were more common in the south-east sections of Lodestone Reef and Helix Reef; however, whether this is related to habitat characteristics or individual variability is unclear as individuals in this section were not detected elsewhere. For all mixed-effects models, the random factor (individual) accounted for ~50–60% of variation.
Summary of monthly mean (±SE) proportions of detections occurring <20 and >20 m for P. leopardus and P. laevis (Helix Reef and Lodestone Reef pooled). Mean proportions of deep/shallow detections were calculated within each 2-h centres of activity period for each individual before averaging for final results. The numbers above each estimate number of individuals with detections for that month
Discussion
Identifying and comparing ecological-based traits, such as habitat preferences and home range areas, help delineate how sympatric fish species access and partition resources (Chin et al. 2012; Espinoza et al. 2015b). This knowledge is important for developing directed management plans because it informs species-specific patterns of vulnerability both spatially and temporally. Plectropomus species form an integral part of fisheries in the Great Barrier Reef and Indo-Pacific region, but their behavioural interactions and long-term reef-use patterns are not well known, especially for species other than P. leopardus. Passive acoustic telemetry provided long-term patterns of space use and activity of co-occurring P. leopardus and P. laevis. Different fine-scale seasonal and diel depth use patterns by each species were demonstrated; however, in general, vertical space use was similar. In contrast, horizontal space use differed between species at several temporal scales revealing behavioural differences between species indicative of niche separation. This study provides a preliminary examination of space use patterns for these co-existing species and helps to inform how each exploits resources on the reef.
Horizontal space use varied between species indicating differences in the amount of reef habitat used. P. laevis was detected on ~2–3 more receivers each month than P. leopardus and hKUD size was ~2 times larger for both core home range and extent at all temporal scales (i.e. weekly, monthly, all detections). P. laevis also moved between receivers along the reef slope more readily than P. leopardus. Broader horizontal space use by P. laevis is indicative of species-specific behavioural, energetic, and/or physiological requirements. In support of this concept, P. laevis were on average ~125 mm larger than P. leopardus; therefore, they may be more energetically driven to find prey resulting in broader movements (Schoener 1968). However, even small P. laevis (<500 mm, FL) were detected on >4 receivers, and there was considerable fish size overlap between species, suggesting other factors may be responsible for the observed movement patterns. For example, the bright colouration of smaller P. laevis (i.e. footballer phase—see Heupel et al. 2010) may increase conspicuousness, making ambush predation less effective compared to sit and wait predation of more cryptic, similar sized P. leopardus. Dietary information for P. laevis is limited, but Matley et al. (2016) found that the isotopic niche space of P. laevis differed significantly from that of P. leopardus and that P. laevis of all sizes likely fed on benthic prey to a greater extent. This further suggests that feeding regime (and any associated interactions) is a strong factor driving differences in space use between species.
As stated above, the size of individuals within and across species often influences activity patterns to optimise energetic budgeting; for example, larger fish swim greater distances to find food or smaller fish remain local to reduce costs of movements (Nash et al. 2015). In this study, 95% hKUDs (i.e. home range extent) were positively correlated to the size of P. laevis demonstrating a higher proclivity for larger individuals to travel outside of core area home ranges (i.e. 50% hKUDs). The lack of seasonal changes in horizontal space use suggests this behaviour is independent of spawning or temperature and may be foraging-based. Alternatively, larger individuals may spend less time seeking refuge from potential predators enabling them to explore further from home sites. Nevertheless, increased roaming by large P. laevis only occurred for home range extent and did not influence the size of core area space use. Similarly, home range (core area and extent) sizes were not affected by the size of P. leopardus. Therefore, independent of fish size and at the spatial resolution of the acoustic array, each species exploited consistent local (core area) horizontal areas. Although not directly tested, the sex of individuals (difficult to assess without lethal sampling) likely did not affect broad patterns of vertical and horizontal space use because sizes of both species are roughly correlated to sexual stage (Ferreira 1995; Heupel et al. 2010).
Vertical movements along the reef slope were common for both species. It was apparent that P. leopardus and P. laevis shifted their depth use daily and seasonally. During the day, P. leopardus were shallower than at night, which differed from P. leopardus at Heron Island (shallower depth use during the night; Matley et al. 2015) indicating patterns vary by location or based on interactions with other species. Plectropomus leopardus are mainly sedentary at night seeking refuge among reef structure (Zeller 1997), consequently differences between studies are likely a reflection of suitable resting habitat. Similarly, the reduced movement between receivers and lower variation in depth use by P. laevis at night further supports increased resting behaviour at night. Caution must be applied when comparing daytime and night-time detections, however, because receiver detection efficiency is often reduced during the night (Payne et al. 2010). Vertical movements revealed seasonal patterns with detections generally deeper in spring for both species. Deeper movements during this period may have been associated with reproductive behaviours as they often occurred during documented spawning periods (at dusk during new moon periods between September to December; Samoilys and Squire 1994; Ferreira 1995; Samoilys 1997; Zeller 1998) and has been postulated previously (see Matley et al. 2015). However, occurrences of deep movements varied daily and seasonally between individuals of both species indicating a lack of synchronicity in this behaviour. Also, season was not a significant factor when only 5-day new moon periods were used, suggesting movements >20 m deep were not strictly driven by spawning or that spawning behaviours are not limited to these periods. Carter et al. (2014) determined that spawning occurred every few days throughout the spawning season at reefs off Townsville, and there is evidence of regional variation in spawning season. Therefore, behavioural patterns of Plectropomus spp. associated with reproduction may still be associated with new moon periods but likely are not restricted to a 5-day period. It is suggested that the function of current fishery closures in the Great Barrier Reef Marine Park (5-day new moon period in October and November) undergo further assessment to evaluate whether closures specifically protect from over-exploitation during spawning or whether they unnecessarily limit fishing activities.
Depth use throughout the day showed species differences with P. leopardus remaining deeper and changing depths less frequently than P. laevis. These differences may be associated with more dedicated search for food by P. laevis, dietary specialisation, and/or niche partitioning/competition (Davis et al. 2015). Alternatively, preferred depth ranges may differ due to species-specific adaptations to metabolic costs associated with daily energy expenditure (Nilsson et al. 2009). Specifically, the energetic cost of greater space use by P. laevis may be reduced by inhabiting warmer waters near the surface, thus optimising metabolic scope (Johansen et al. 2013; 2015). This may also explain why P. laevis moved between receivers more readily during warmer months. The reduced energetic cost of restricted movement by P. leopardus may enable individuals to remain in deeper, cooler habitats. Overall, depth differences between species were notably small (usually <5 m) and species commonly shared the same space along the reef slope, suggesting similar habitat selection. For sympatric species to co-exist, each typically has to exploit an alternate habitat or resource (Connell 1980). Different home ranges and dietary patterns may provide the resource partitioning needed for these species to survive together; however, direct observations of interactions between species (e.g. competition or territorial/aggressive behaviour) and extensive testing are needed to validate this.
Fishing pressure on ‘coral trout’ is focused on shallow sections of reef (~85% of fishing effort; Little et al. 2008) to reduce barotrauma for live-trade. Thus, when deeper waters are selected by either species, the risk of capture inevitably decreases. In this study, both P. laevis and P. leopardus were caught <20 m deep, and ~75% of detections were within this depth range, indicating both species are consistently vulnerable to capture. It is unclear if broader horizontal movements of P. laevis increase or decrease fishery encounters or if their removal has larger reef-scale implications. Better understanding of spatial and temporal behavioural trends in congenerics will better equip fishers for more efficient operation and/or managers to develop successful regulations (e.g. identifying temporal or spatial exploitation risk).
A relatively large number of P. leopardus were not included in the analyses (~35% and ~50% at Helix Reef and Lodestone Reef, respectively) due to limited detections. Although some individuals had detection periods >15 days (e.g. detected on reef flat) or were reported captured by fishers, the majority of these individuals were only detected for a few days (and not often), leaving uncertainty as to why. The lack of data could not be attributed to the size of fish, reef, or location. The release distance from a receiver also did not affect inclusion although a release distance <200 m is suggested to maximise probability of detection. This loss of individuals is not necessarily surprising considering the receiver array was originally designed for inter-reef mobile predators. The rarity of P. leopardus inter-reef movement (Davies 1996; Sumpton et al. 2008) and broad receiver coverage at Helix Reef suggests individuals do not move far regardless of dietary, physiological, reproductive, and/or refuging needs. Considering this high residency, it is more likely that individuals were nearby but simply outside detection ranges, transmissions were blocked due to structural complexity of coral reef habitat, or individuals had died. A denser receiver array designed specifically for more sedentary reef fish is required to better understand the behaviour of all individuals in a population (Currey et al. 2015b).
This study used acoustic telemetry to show that two congenerics—often grouped together in research and management, have distinct activity patterns on coral reefs, mainly, P. laevis was more mobile than P. leopardus, using up to half of Helix Reef. Also, P. laevis typically remained shallower than P. leopardus, and both were less vulnerable to capture (i.e. >20 m deep) in warmer months. Unfortunately, sample sizes of P. laevis were small and consequently, these results are preliminary. Nevertheless, these findings, in conjunction with dietary segregation (Matley et al. 2016), indicate that resource use varies between species. As a result, environmental changes and human exploitation will likely impact each species differently. Management decisions will be more effective if they account for species differences relating to behaviour or ecology, as well as life history parameters. It is unclear if behavioural differences are driven by competitive interactions, energetic/foraging constraints, or a combination thereof and should be considered in future research. Based on these findings P. laevis may be more vulnerable than P. leopardus to over-fishing or environmental stressors due to its larger size, lower abundance, and greater energetic requirements.
References
Ayling AM, Choat JH (2008) Abundance patterns of reef sharks and predatory fishes on differently zoned reefs in the offshore Townsville region: final report to the Great Barrier Reef Marine Park Authority, Research Publication No. 91, Great Barrier Reef Marine Park Authority, Townsville
Barton K (2013) MuMIn: multi-model inference. R package Version 1.9.13. http://CRAN.R-project.org/package=MuMIn
Bates D, Maechler M, Bolker BM, Walker S (2014) lme4: linear mixed-effects models using Eigen and S4. R package version 1.0-6. http://cran.r-project.org/package=lme4
Beaman RJ (2010) Project 3DGBR: a high-resolution depth model for the Great Barrier Reef and Coral Sea. In Marine and Tropical Sciences Research Facility (MTSRF)
Boaden AE, Kingsford MJ (2015) Predators drive community structure in coral reef fish assemblages. Ecosphere 6:1–33
Botsford L, Castilla J, Peterson C (1997) The management of fisheries and marine ecosystems. Science 277:509–515
Braune BM, Gaston AJ, Hobson KA, Gilchrist HG, Mallory ML (2014) Changes in food web structure alter trends of mercury uptake at two seabird colonies in the Canadian Arctic. Environ Sci Technol 48:13246–13252. doi:10.1021/es5036249
Bunt CM, Kingsford MJ (2014) Movement, habitat utilization and behaviour of coral trout Plectropomus leopardus during and after the reproductive period on the southern Great Barrier Reef. Mar Ecol Prog Ser 496:33–45
Calenge C (2006) The package adehabitat for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519
Carter AB, Russ GR, Tobin AJ, Williams AJ, Davies CR, Mapstone BD (2014) Spatial variation in the effects of size and age on reproductive dynamics of common coral trout Plectropomus leopardus. J Fish Biol 84:1074–1098
Chin A, Tobin A, Simpfendorfer C, Heupel M (2012) Reef sharks and inshore habitats: patterns of occurrence and implications for vulnerability. Mar Ecol Prog Ser 460:115–125
Connell JH (1980) Diversity and the coevolution of competitors, or the ghost of competition past. Oikos 35:131–138
Currey LM, Heupel MR, Simpfendorfer CA, Williams AJ (2015a) Assessing fine-scale diel movement patterns of an exploited coral reef fish. Anim Biotelem 3:1–13
Currey LM, Heupel MR, Simpfendorfer CA, Williams AJ (2015b) Assessing environmental correlates of fish movement on a coral reef. Coral Reefs 34:1267–1277
Dance M, Rooker J (2015) Habitat- and bay-scale connectivity of sympatric fishes in an estuarine nursery. Estuar Coast Shelf Sci 167:447–457
Davies CR (1996) Inter-reef movement of the common coral trout, Plectropomus leopardus. Great Barrier Reef Marine Park Authority Research Publication No. 61. GBRMPA, Townsville
Davis WT, Drymon JM, Powers SP (2015) Spatial and dietary overlap creates potential for competition between red snapper (Lutjanus campechanus) and vermilion snapper (Rhomboplites aurorubens). PLoS ONE 10:e0144051
Emslie MJ, Logan M, Williamson DH, Ayling AM, MacNeil AM, Ceccarelli D, Cheal AJ, Evans RD, Johns KA, Jonker MJ, Miller IR, Osborne K, Russ GR, Sweatman HP (2015) Expectations and outcomes of reserve network performance following re-zoning of the Great Barrier Reef Marine Park. Curr Biol 25:983–992
Espinoza M, Heupel MR, Tobin AJ, Simpfendorfer CA (2015a) Movement patterns of silvertip sharks (Carcharhinus albimarginatus) on coral reefs. Coral Reefs 34:807–821. doi:10.1007/s00338-015-1312-0
Espinoza M, Munroe SEM, Clarke TM, Fisk AT, Wehrtmann IS (2015b) Feeding ecology of common demersal elasmobranch species in the Pacific coast of Costa Rica inferred from stable isotope and stomach content analyses. J Exp Mar Biol Ecol 470:12–25
Estes JA, Terborgh J, Brashares JS, Power ME, Berger J, Bond WJ, Carpenter SR, Essington TE, Holt RD, Jackson JBC, Marquis RJ, Oksanen L, Oksanen T, Paine RT, Pikitch EK, Ripple WJ, Sandin SA, Scheffer M, Schoener TW, Shurin JB, Sinclair ARE, Soulé ME, Virtanen R, Wardle DA (2011) Trophic downgrading of planet Earth. Science 333:301–306
Ferreira BP (1995) Reproduction of the common coral trout Plectropomus leopardus (Serranidae: Epinephelinae) from the central and northern Great Barrier Reef, Australia. Bull Mar Sci 56:653–669
Gleiss AC, Wright S, Liebsch N, Wilson RP, Norman B (2013) Contrasting diel patterns in vertical movement and locomotor activity of whale sharks at Ningaloo Reef. Mar Biol 160:2981–2992
Goeden GB (1978) A monograph of the coral trout Plectropomus leopardus. Qld Fish Serv Res Bull 1:1–42
Graham NAJ, Evans RD, Russ GR (2003) The effects of marine reserve protection on the trophic relationships of reef fishes on the Great Barrier Reef. Environ Conserv 30:200–208
Guzzo MM, Blanchfield PJ, Chapelsky AJ, Cott PA (2015) Resource partitioning among top-level piscivores in a sub-Arctic lake during thermal stratification. J Great Lakes Res 42:276–285
Heithaus MR, Frid A, Wirsing AJ, Worm B (2008) Predicting ecological consequences of marine top predator declines. Trends Ecol Evol 23:202–210
Heupel MR, Williams AJ, Welch DJ, Davies CR, Adams S, Carlos G, Mapstone BD (2010) Demography of a large exploited grouper, Plectropomus laevis: implications for fisheries management. Mar Freshw Res 61:184–195
Hill NJ, Tobin AJ, Reside AE, Pepperell JG, Bridge TCL (2016) Dynamic habitat suitability modelling reveals rapid poleward distribution shift in a mobile apex predator. Glob Change Biol 22:1086–1096
Hussey NE, Kessel ST, Aarestrup K, Cooke SJ, Cowley PD, Fisk AT, Harcourt RG, Holland KN, Iverson SJ, Kocik JF, Mills Flemming JE, Whoriskey FG (2015) Aquatic animal telemetry: a panoramic window into the underwater world. Science 348:1255642
Huveneers C, Simpfendorfer CA, Kim S, Semmens J, Hobday AJ, Pederson H, Stieglitz T, Vallee R, Webber D, Heupel MR, Peddemors V, Harcourt R (2016) The influence of environmental parameters on the performance and detection range of acoustic receivers. Methods Ecol Evol 7:825–835
Johansen JL, Messmer V, Coker DJ, Hoey AS, Pratchett MS (2013) Increasing ocean temperatures reduce activity patterns of a large commercially important coral reef fish. Glob Change Biol 20:1067–1074
Johansen JL, Pratchett MS, Messmer V, Coker DJ, Tobin AJ, Hoey AS (2015) Large predatory coral trout species unlikely to meet increasing energetic demands in a warming ocean. Sci Rep 5:13830. doi:10.1038/srep13830
Kessel ST, Cooke SJ, Heupel MR, Hussey NE, Simpfendorfer CA, Vagle S, Fisk AT (2014) A review of detection range testing in aquatic passive acoustic telemetry studies. Rev Fish Biol Fish 24:199–218
Leigh GM, Campbell AB, Lunow CP, O’Neill MF (2014) Stock assessment of the Queensland east coast common coral trout (Plectropomus leopardus) fishery. Queensland Government, Brisbane. http://era.deedi.qld.gov.au/4547
Little LR, Smith ADM, Mcdonald AD (2005) Effects of size and fragmentation of marine reserves and fisher infringement on the catch and biomass of coral trout, Plectropomus leopardus, on the Great Barrier Reef, Australia. Fish Manag Ecol 12:177–188
Little LR, Begg GA, Goldman B, Ellis N, Mapstone BD, Punt AE, Jones A, Sutton S, Williams A (2008) Modelling multi-species targeting of fishing effort in the Queensland Coral Reef Fin Fish Fishery. Fishing and Fisheries Research Centre Technical Report No. 2. Fishing and Fisheries Research Centre, James Cook University, Townsville
Mapstone BD, Davies CR, Little LR, Punt AE, Smith ADM, Pantus F, Lou DC, Williams AJ, Jones A, Ayling AM, Russ GR, McDonald AD (2004) The effects of line fishing on the Great Barrier Reef and evaluation of alternative potential management strategies. CRC Reef Research Centre Technical Report No. 52, CRC Reef Research Centre, Townsville
Matley JK, Heupel MR, Simpfendorfer CA (2015) Depth and space use of leopard coralgrouper Plectropomus leopardus using passive acoustic tracking. Mar Ecol Prog Ser 521:201–216
Matley JK, Tobin AJ, Simpfendorfer CA, Fisk AT, Heupel MR (2016) Trophic niche and spatio-temporal changes in the feeding ecology of two sympatric species of coral trout (Plectropomus leopardus and P. laevis). Mar Ecol Prog Ser. doi:10.3354/meps11971
McLean DL, Harvey ES, Meeuwig JJ (2011) Declines in the abundance of coral trout (Plectropomus leopardus) in areas closed to fishing at the Houtman Abrolhos Islands, Western Australia. J Exp Mar Biol Ecol 406:71–78
Mellin C, Mouillot D, Kulbicki M, McClanahan T, Vigliola L, Bradshaw C, Brainard R, Chabanet P, Edgar G, Fordham D, Friedlander A, Parravicini V, Sequeira A, Stuart-Smith R, Wantiez L, Caley M (2016) Humans and seasonal climate variability threaten large-bodied coral reef fish with small ranges. Nat Commun 7:10491. doi:10.1038/ncomms10491
Mueller A-K, Chakarov N, Heseker H, Krüger O (2016) Intraguild predation leads to cascading effects on habitat choice, behaviour and reproductive performance. J Anim Ecol 85:774–784
Nash KL, Welsh JQ, Graham NAJ, Bellwood DR (2015) Home-range allometry in coral reef fishes: comparison to other vertebrates, methodological issues and management implications. Oecologia 177:73–83
Newton K, Côté IM, Pilling GM, Jennings S, Dulvy NK (2007) Current and future sustainability of island coral reef fisheries. Curr Biol 17:655–658
Nilsson GE, Crawley N, Lunde IG, Munday PL (2009) Elevated temperature reduces the respiratory scope of coral reef fishes. Glob Change Biol 15:1405–1412
Payne N, Gillanders B, Webber D, Semmens J (2010) Interpreting diel activity patterns from acoustic telemetry: the need for controls. Mar Ecol Prog Ser 419:295–301
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team (2013) nlme: linear and nonlinear mixed effects models. R package v. 3.1-109
R Core Team (2016) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rizzari JR, Frisch AJ, Hoey AS, McCormick MI (2014) Not worth the risk: apex predators suppress herbivory on coral reefs. Oikos 123:829–836
Roessig J, Woodley C, Cech J, Hansen L (2004) Effects of global climate change on marine and estuarine fishes and fisheries. Rev Fish Biol Fish 14:251–275
Sadovy Y (2005) Trouble on the reef: the imperative for managing vulnerable and valuable fisheries. Fish Fish 6:167–185
Sadovy de Mitcheson Y, Colin PL (2012) Reef fish spawning aggregations: biology, research and management. In: Noakes D (ed) Fish & fisheries series, vol 35. Springer, Dordrecht
Sadovy de Mitcheson Y, Craig MT, Bertoncini AA, Carpenter KE, Cheung WWL, Choat JH, Cornish AS, Fennessy ST, Ferreira BP, Heemstra PC, Liu M, Myers RF, Pollard DA, Rhodes KL, Rocha LA, Russell BC, Samoilys MA, Sanciangco J (2013) Fishing groupers towards extinction: a global assessment of threats and extinction risks in a billion dollar fishery. Fish Fish 14:119–136
Samoilys MA (1997) Movement in a large predatory fish: coral trout, Plectropomus leopardus (Pisces: Serranidae), on Heron Reef, Australia. Coral Reefs 16:151–158
Samoilys MA, Squire LC (1994) Preliminary observations on the spawning behaviour of coral trout, Plectropomus leopardus (Pisces: Serranidae), on the Great Barrier Reef. Bull Mar Sci 54:332–342
Schoener TW (1968) Sizes of feeding territories among birds. Ecology 49:123–141
Simpfendorfer CA, Heupel MR, Hueter RE (2002) Estimation of short-term centers of activity from an array of omnidirectional hydrophones and its use in studying animal movements. Can J Fish Aquat Sci 59:23–32
St. John J (2001) Temporal variation in the diet of a coral reef piscivore (Pisces: Serranidae) was not seasonal. Coral Reefs 20:163–170
Sumpton W, Mayer D, Brown I, Sawynok B, McLennan M, Butcher A, Kirkwood J (2008) Investigation of movement and factors influencing post-release survival of line-caught coral reef fish using recreational tag-recapture data. Fish Res 92:189–195
Tobin A, Schlaff A, Tobin R, Penny A, Ayling T, Ayling A, Krause B, Welch D, Sutton S, Sawynok B, Marshall N, Marshall P, Maynard J (2010) Adapting to change: minimising uncertainty about the effects of rapidly-changing environmental conditions on the Queensland Coral Reef Fin Fish Fishery. Final Report to the Fisheries Research & Development Corporation, Project 2008/103. Fishing & Fisheries Research Centre Technical Report no. 11, James Cook University, Townsville
Tobin A, Currey L, Simpfendorfer C (2013) Informing the vulnerability of species to spawning aggregation fishing using commercial catch data. Fish Res 143:47–56
Waldie PA, Almany GR, Sinclair-Taylor TH, Hamilton RJ, Potuku T, Priest MA, Rhodes KL, Robinson J, Cinner JE, Berumen ML (2016) Restricted grouper reproductive migrations support community-based management. R Soc Open Sci 3:150694
Warnes GR, Bolker B, Lumley T, Johnson RC (2015). gmodels: various R programming tools for model fitting. R package v. 2.16.2
Zaret TM, Rand AS (1971) Competition in tropical stream fishes: support for the competitive exclusion principle. Ecology 52:336–342
Zeller D (1997) Home range and activity patterns of the coral trout Plectropomus leopardus (Serranidae). Mar Ecol Prog Ser 154:65–77
Zeller DC (1998) Spawning aggregations: patterns of movement of the coral trout Plectropomus leopardus (Serranidae) as determined by ultrasonic telemetry. Mar Ecol Prog Ser 162:253–263
Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
This project was funded by the Australian Government’s National Environmental Research Program (Tropical Ecosystems Hub Project 6.1). MRH was supported by an Australian Research Council Future Fellowship (#FT100101004). Additional funding was granted to JKM from the James Cook University College of Marine and Environmental Sciences and the Graduate Research School; as well as a Natural Sciences and Engineering Research Council (NSERC) PGS D scholarship. All research was conducted under JCU Animal Ethics Permit A1933 and research permits from the Great Barrier Reef Marine Park Authority (G12/35236.1 and G14/36624.1) and Queensland Department of Primary Industries and Fisheries (144482). The authors thank crew from the RV James Kirby, Vemco staff—particularly Courtney MacSween, as well as JCU staff/students who helped tag fish and download receivers including S. Moore, F. de Faria, P. Yates, C. Aguilar Hurtado, G. Molinaro, S. Sherman, and M. Espinoza.
Funding
This project was funded by the Australian Government’s National Environmental Research Program (Tropical Ecosystems Hub Project 6.1). MRH was supported by an Australian Research Council Future Fellowship (#FT100101004). Additional funding was granted to JKM from the James Cook University College of Marine and Environmental Sciences and the Graduate Research School; as well as a Natural Sciences and Engineering Research Council (NSERC) PGS D scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All research was conducted under JCU Animal Ethics Permit A1933 and research permits from the Great Barrier Reef Marine Park Authority (G12/35236.1 and G14/36624.1) and Queensland Department of Primary Industries and Fisheries (144482).
Additional information
Responsible Editor: K.D. Clements.
Reviewed by Undisclosed experts.
Rights and permissions
About this article
Cite this article
Matley, J.K., Tobin, A.J., Lédée, E.J.I. et al. Contrasting patterns of vertical and horizontal space use of two exploited and sympatric coral reef fish. Mar Biol 163, 253 (2016). https://doi.org/10.1007/s00227-016-3023-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-3023-7