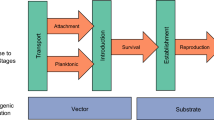
Abstract
Species introductions have increased dramatically in number, rate, and magnitude of impact in recent decades. In marine systems, invertebrates are the largest and most diverse component of coastal invasions throughout the world. Ascidians are conspicuous and well-studied members of this group, however, much of what is known about their invasion history is limited to particular species or locations. Here, we provide a large-scale assessment of invasions, using an extensive literature review and standardized field surveys, to characterize the invasion dynamics of non-native ascidians in the continental United States and Alaska. Twenty-six non-native ascidian species have established documented populations on the Pacific, Atlantic and Gulf coasts (spanning 25–57°N). Invader species richness is greatest for the Pacific coast (19 spp.), followed by the Atlantic (14 spp.) and Gulf (6 spp.) coasts, and decreases towards higher latitudes. Most species (97 %) expanded their range after initial introduction, although the direction and latitudinal extent of secondary spread varied. Temporal analyses, based on literature reported first records and repeated field surveys, show an increase in recorded non-native ascidians at continental, regional, and local scales. Our results underscore that non-native species continue to establish and spread, and the transfer of biofouling organisms on underwater surfaces of vessels is an active and potent vector that remains largely unmanaged. More broadly, we suggest that ascidians provide a tractable and important indicator group for evaluating invasion dynamics and management strategies.






Similar content being viewed by others
References
Adams CM, Shumway SE, Whitlatch RB, Getchis T (2011) Biofouling in marine molluscan shellfish aquaculture: a survey assessing the business and economic implications of mitigation. J World Aquac Soc 42:242–252
Airoldi L, Turon X, Perkol-Finkel S, Rius M (2015) Corridors for aliens but not for natives: effects of marine urban sprawl at a regional scale. Divers Distrib 21:755–768
Aldred N, Clare AS (2014) Mini-review: impact and dynamics of surface fouling by solitary and compound ascidians. Biofouling 30:259–270
Berman J, Harris L, Lambert W, Buttrick M, Dufresne M (1992) Recent invasions of the Gulf of Maine: three contrasting ecological histories. Conserv Biol 6:435–441
Bingham BL (1992) Life histories in an epifaunal community: coupling of adult and larval processes. Ecology 73:2244–2259
Blackburn TM, Pysek P, Bacher S, Carlton JT, Duncan RP, Jarosik V, Wilson JR, Richardson DM (2011) A proposed unified framework for biological invasions. Trends Ecol Evol 26:333–339
Blum JC, Chang AL, Liljesthröm M, Schenk ME, Steinberg MK, Ruiz GM (2007) The non-native solitary ascidian Ciona intestinalis depresses species richness. J Exp Mar Biol Ecol 342:5–14
Bock DG, Zhan A, Lejeusne CL, MacIsaac HJ, Cristescu ME (2011) Looking at both sides of the invasion: patterns of colonization in the violet tunicate Botrylloides violaceus. Mol Ecol 20:503–516
Bouchemousse S, Bishop JDD, Viard F (2016) Contrasting global genetic patterns of two biologically similar, widespread and invasive Ciona species (Tunicata, Ascidiacea). Sci Rep 6:24875
Brunetti R, Gissi R, Pennati R, Caicci F, Gasparini F, Manni L (2015) Morphological evidence that the molecularly determined Ciona intestinalis type A and type B are different species: Ciona robusta and Ciona intestinalis. J Zool Syst Evol Res 53:186–193
Bullard SG, Lambert G, Carman MR, Byrnes J, Whitlatch RB, Ruiz G, Miller RJ, Harris L, Valentine PC, Collie JS, Pederson J, McNaught DC, Cohen AN, Asch RG, Dijkstra J, Heinonen K (2007) The colonial ascidian Didemnum sp. a: current distribution, basic biology and potential threat to marine communities of the northeast and west coasts of North America. J Exp Mar Biol Ecol 342:99–108
California State Lands Commission (2015) Biofouling management to minimize the transfer of nonindigenous species from vessels operating in California waters. Proposed Regulatory Notice, July 31, 2015. In: Division CSLCMF (ed) Article 4.8, Sacramento, California, pp 15
Callahan AG, Deibel D, McKenzie CH, Hall JR, Rise ML (2010) Survey of harbours in Newfoundland for indigenous and non-indigenous ascidians and an analysis of their cytochrome c oxidase I gene sequences. Aquat Invasions 5:31–39
Carlton JT (1979) History, biogeography, and ecology of the introduced marine and estuarine invertebrates of the Pacific Coast of North America. University of California, Davis
Carlton JT (1989) Man’s role in changing the face of the ocean: biological invasions and implications for conservation of near-shore environments. Conserv Biol 3:265–273
Carlton JT, Geller JB (1993) Ecological roulette: the global transport of nonindigenous marine organisms. Science 261:78–82
Carlton JT, Hodder J (1995) Biogeography and dispersal of coastal marine organisms: experimental studies of a replica of a 16th-century sailing vessel. Mar Biol 121:721–730
Carman MR, Grunden DW (2010) First occurrence of the invasive tunicate Didemnum vexillum in eelgrass habitat. Aquat Invasions 5:23–29. doi:10.3391/ai.2010.5.1.4
Carman MR, Morris JA, Karney RC, Grunden DW (2010) An initial assessment of native and invasive tunicates in shellfish aquaculture of the North American east coast. J Appl Ichthyol 26:8–11
Carver CE, Chisholm A, Mallet AL (2003) Strategies to mitigate the impacts of Ciona intestinalis biofouling on shellfish production. J Shellfish Res 22:521–631
Castilla JC, Guinez R, Caro AU, Ortiz V (2004) Invasion of a rocky intertidal shore by the tunicate Pyura praeputialis in the Bay of Antofagasta, Chile. Proc Natl Acad Sci USA 101:8517–8524
Clarke Murray C, Pakhomov EA, Therriault TW (2011) Recreational boating: a large unregulated vector transporting marine invasive species. Divers Distrib 17:1161–1172
Cohen AN (2005) Guide to the exotic species of San Francisco. http://www.exoticsguide.org/
Cohen AN, Carlton JT (1998) Accelerating invasion rate in a highly invaded estuary. Science 279:555–558
Cohen CS, McCann L, Davis T, Shaw L, Ruiz G (2011) Discovery and significance of the colonial tunicate Didemnum vexillum in Alaska. Aquat Invasions 6:263–271
Coutts ADM, Dodgshun TJ (2007) The nature and extent of organisms in vessel sea-chests: a protected mechanism for marine bioinvasions. Mar Pollut Bull 54:875–886
Culbertson J, Harper D (2000) Settlement of a colonial ascidian on an artificial reef in the Gulf of Mexico. In: Mckay M, Nides J, Vigil D (eds) Proceedings of Gulf of Mexico fish and fisheries: bringing together new and recent research. OCS Reports, US Minerals Management Service, no 2002–2004, New Orleans, LA, pp 614–630
Dalby JEJ, Young CM (1992) Role of early post-settlement mortality in setting the upper depth limit of ascidians in Florida epifaunal communities. Mar Ecol Prog Ser 80:221–228
Darling JA, Herborg L-M, Davidson IC (2012) Intracoastal shipping drives patterns of regional population expansion by an invasive marine invertebrate. Ecol Evol 2:2552–2561
Davidson IC, Simkanin C (2012) The biology of ballast water 25 years later. Biol Invasions 14:9–13
Davidson IC, Zabin CJ, Chang AL, Brown CW, Sytsma MD, Ruiz GM (2010) Recreational boats as potential vectors of marine organisms at an invasion hotspot. Aquat Biol 11:179–191
Davidson I, Scianni C, Hewitt C, Everett R, Holm E, Tamburri M, Ruiz G (2016) Mini-review: assessing the drivers of ship biofouling management—aligning industry and biosecurity goals. Biofouling 32:411–428
de Rivera CE, Steves BD, Fofonoff PW, Hines AH, Ruiz GM (2011) Potential for high-latitude marine invasions along western North America. Divers Distrib 17:1198–1209
Dijkstra JA, Harris LG (2009) Maintenance of diversity altered by a shift in dominant species: implications for species coexistence. Mar Ecol Prog Ser 387:71–80
Dijkstra JA, Nolan R (2011) Potential of the invasive colonial ascidian, Didemnum vexillum, to limit escape response of the sea scallop, Placopecten magellanicus. Aquat Invasions 6:451–456
Dijkstra JA, Westerman EL, Harris LG (2011) The effects of climate change on species composition, succession and phenology: a case study. Glob Change Biol 17:2360–2369
Dumont CP, Gaymer CF, Thiel MT (2011) Predation contributes to invasion resistance of benthic communities against the non-indigenous tunicate Ciona intestinalis. Biol Invasions 13:2023–2034
Eldredge LG (1966) A taxonomic review of Indo-Pacific didemnid ascidians and descriptions of twenty-three central Pacific species. Micronesica 2:161
Fletcher LM, Forrest BM, Bell JJ (2012) Natural dispersal mechanisms and dispersal potential of the invasive ascidian Didemnum vexillum. Biol Invasions 15:627–643
Fofonoff PW, Ruiz GM, Steves B, Carlton JT (2003) In ships or on ships? Mechanisms of transfer and invasion for nonnative species to the coasts of North America. In: Ruiz GM, Carlton JT (eds) Invasive species: vector and management strategies. Island Press, Washington, D.C., pp 152–182
Forrest BM, Fletcher LM, Atalah J, Piola RF, Hopkins GA (2013) Predation limits spread of Didemnum vexillum into natural habitats from refuges on anthropogenic structures. PLoS ONE 8:e82229
Frey MA, Simard N, Robichaud DD, Martin JL, Therriault TW (2014) Fouling around: vessel sea-chests as a vector for the introduction and spread of aquatic invasive species. Manag Biol Invasions 5:21–30
Glasby TM, Connell SD, Holloway MG, Chad LH (2007) Nonindigenous biota on artificial structures: could habitat creation facilitate biological invasions? Mar Biol 151:887–895
Goldstien SJ, Dupont L, Viard F, Hallas PJ, Nishikawa T, Schiel DR, Gemmell NJ, Bishop JD (2011) Global phylogeography of the widely introduced North West Pacific ascidian Styela clava. PLoS ONE 6:e16755
Grosholz ED, Crafton RE, Fontana RE, Pasari JR, Williams SL, Zabin CJ (2015) Aquaculture as a vector for marine invasions in California. Biol Invasions 17:1471–1484
Lambert G (2002) Nonindigenous ascidians in tropical waters. Pac Sci 56:191–298
Lambert G (2005a) Ecology and natural history of the protochordates. Can J Zool 83:34–50
Lambert G (2005b) First North American record of the ascidian Perophora japonica. J Mar Biol Assoc UK 85:1011–1012
Lambert G (2007a) Invasive sea squirts: a growing global problem. J Exp Mar Biol Ecol 342:3–4
Lambert G (2007b) The nonindigenous ascidian Molgula ficus in California. Cah Biol Mar 48:95–102
Lambert G (2009) Adventures of a sea squirt sleuth: unraveling the identity of Didemnum vexillum, a global ascidian invader. Aquat Invasions 4:5–28
Lambert CC, Lambert G (1998) Non-indigenous ascidians in southern California harbors and marinas. Mar Biol 130:675–688
Lambert CC, Lambert G (2003) Persistence and differential distribution of nonindigenous ascidians in harbors of the southern California Bight. Mar Ecol Prog Ser 259:145–161
Lambert G, Faulkes Z, Lambert CC, Scofield VL (2005) Ascidians of South Padre Island, Texas, with a key to species. Tex J Sci 57:251–262
Lambert G, Shenkar N, Swalla BJ (2010) First Pacific record of the north Atlantic ascidian Molgula citrina—bioinvasion or circumpolar distribution? Aquat Invasions 5:369–378
LeBlanc AR, Landry T, Miron G (2003) Fouling organisms of the blue mussel Mytilus edulis: their effect on nutrient uptake and release. J Shellfish Res 22:633–638
LeGresley MM, Martin JL, McCurdy P, Thorpe B, Chang BD (2008) Nonindigenous tunicate species in the Bay of Fundy, eastern Canada. ICES J Mar Sci 65:770–774
Lutzen J (1999) Styela clava Herdman (Urochordata, Ascidiacea), a successful immigrant to North West Europe: ecology, propagation and chronolgy of spread. Helgo Wiss Meeresunters 52:383–391
Ma K, Deibel D, McKenzie CH (2011) Indigenous and non-indigenous ascidian tunicates of Newfoundland and Labrador. AAC Adv Spec Publ 17:58–63
MacGinitie GE, MacGinitie N (1968) Natural history of marine animals. McGraw-Hill, New York
Massachusetts Office of Coastal Zone Management C (2013) Report on the 2010 Rapid Assessment Survey of Marine Species at New England Floating Docks and Rocky Shores. Executive Office of Energy and Environmental Affairs, Office of Coastal Zone Management, Boston, Massachusetts
McCann LD, Holzer KK, Davidson IC, Ashton GV, Chapman MD, Ruiz GM (2013) Promoting invasive species control and eradication in the sea: options for managing the tunicate invader Didemnum vexillum in Sitka, Alaska. Mar Pollut Bull 77:165–171
McKindsey CW, Landry T, O’Beirn FX, Davies IM (2007) Bivalve aquaculture and exotic species: a review of ecological considerations and management issues. J Shellfish Res 26:281–294
Millar RH (1971) The biology of ascidians. Adv Mar Biol 9:1–100
Molnar JL, Gamboa RL, Revenga C, Spalding MD (2008) Assessing the global threat of invasive species to marine biodiversity. Front Ecol Environ 6:485–492
Monniot C, Monniot F, Laboute P (1991) Coral reef ascidians of New Caledonia. Orstom, Paris, p 247
Moore AM, Vercaemer B, DiBacco C, Sephton D, Ma KCK (2014) Invading Nova Scotia: first records of Didemnum vexillum Kott, 2002 and four more non-indigenous invertebrates in 2012 and 2013. BioInvasions Rec 3:225–234
Parker IM, Simberloff D, Lonsdale WM, Goodell K, Wonham M, Kareiva PM, Williamson MH, von Holle B, Moyle PB, Byers JE, Goldwasser L (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1:3–19
Pereyra PJ, Narvarte M, Tatián M, González R (2015) The simultaneous introduction of the tunicate Styela clava (Herdman, 1881) and the macroalga Undaria pinnatifida (Harvey) Suringar, 1873, in northern Patagonia. BioInvasions Rec 4:179–184
Peterson JK, Svane I (1995) Larval dispersal in the ascidian Ciona intestinalis (L.). Evidence for a closed population. J Exp Mar Biol Ecol 186:89–102
Preisler RK, Wasson K, Wolff WJ, Tyrrell MC (2009) Invasions of estuaries vs the adjacent open coast: a global perspective. In: Rilov G, Crooks JA (eds) Biological invasions in marine ecosystems. Springer-Verlag, Berlin Heidelberg, pp 587–617
Ramsay A, Davidson J, Landry T, Arsenault G (2008) Process of invasiveness among exotic tunicates in Prince Edward Island, Canada. Biol Invasions 10:1311–1316
Reinhardt JF, Stefaniak LM, Hudson DM, Mangiafico J, Gladych R, Whitlatch RB (2010) First record of the non-native light bulb tunicate Clavelina lepadiformis (Müller, 1776) in the northwest Atlantic. Aquat Invasions 5:185–190
Ritter WE, Forsyth RA (1917) Ascidians of the littoral zone of southern California. University of California, Berkeley
Rius M, Heasman KG, McQuaid CD (2011) Long-term coexistence of non-indigenous species in aquaculture facilities. Mar Pollut Bull 62:2395–2403
Rocha R, Kremer LP, Baptista MS, Metri R (2009) Bivalve cultures provide habitat for exotic tunicates in southern Brazil. Aquat Invasions 4:195–205
Rogers TL, Byrnes JE, Stachowicz JJ (2016) Native predators limit invasion of benthic invertebrate communities in Bodega Harbor, California, USA. Mar Ecol Prog Ser 545:161–173
Roman J, Darling JA (2007) Paradox lost: genetic diversity and the success of aquatic invasions. Trends Ecol Evol 22:455–464
Ruiz GM, Hewitt CL (2009) Latitudinal patterns of biological invasions in marine ecosystems: a polar perspective. In: Krupnik I, Lang MA, Miller SE (eds) Smithsonian at the poles : contributions to International Polar Year science. Smithsonian Institution Scholarly Press, Washington DC, pp 347–358
Ruiz GM, Carlton JT, Grosholz ED, Hines AH (1997) Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent, and consequences. Am Zool 37:621–632
Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH (2000) Invasion of coastal marine communities in North America: apparent patterns, processes, and biases. Annu Rev Ecol Syst 31:481–531
Ruiz GM, Freestone AL, Fofonoff PW, Simkanin C (2009) Habitat distribution and heterogeneity in marine Invasion dynamics: the importance of hard substrate and artificial structure. In: Wahl M (ed) Marine hard bottom communities. Springer, Berlin Heidelberg, pp 321–332
Ruiz GM, Fofonoff PW, Steves B, Foss SF, Shiba SN (2011) Marine invasion history and vector analysis of California: a hotspot for western North America. Divers Distrib 17:362–373
Ruiz GM, Fofonoff PW, Ashton G, Minton MS, Miller AW, Kingsley-Smith PR, Kellogg ML (2013) Geographic variation in marine invasions among large estuaries: effects of ships and time. Ecol Appl 23:311–320
Ruiz GM, Fofonoff PW, Steves BP, Carlton JT (2015) Invasion history and vector dynamics in coastal marine ecosystems: a North American perspective. Aquat Ecosyst Health Manage 18:299–311
Sargent PS, Wells T, Matheson K, McKenzie CH, Deibel D (2013) First record of vase tunicate, Ciona intestinalis (Linnaeus, 1767) in coastal Newfoundland waters. BioInvasions Rec 2:89–98
Sephton D, Vercaemer B, Nicolas JM, Keays J (2011) Monitoring for invasive tunicates in Nova Scotia, Canada (2006–2009). Aquat Invasions 6:391–403
Shenkar N, Swalla BJ (2011) Global diversity of Ascidiacea. PLOS One 6:e20657
Simberloff D, Martin J-L, Genovesi P, Maris V, Wardle DA, Aronson J, Courchamp F, Galil B, Garcıa-Berthou E, Pascal M, Pysek P, Sousa R, Tabacchi E, Vila M (2012) Impacts of biological invasions: what’s what and the way forward. Trends Ecol Evol 28:58–66
Simkanin C, Davidson I, Falkner M, Sytsma M, Ruiz G (2009) Intra-coastal ballast water flux and the potential for secondary spread of non-native species on the US West Coast. Mar Pollut Bull 58:366–374
Simkanin C, Davidson IC, Dower JF, Jamieson G, Therriault TW (2012) Anthropogenic structures and the infiltration of natural benthos by invasive ascidians. Mar Ecol 499:499–511
Simkanin C, Dower JF, Filip N, Jamieson G, Therriault TW (2013) Biotic resistance to the infiltration of natural benthic habitats: examining the role of predation in the distribution of the invasive ascidian Botrylloides violaceus. J Exp Mar Biol Ecol 439:76–83
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdana ZA, Finlayson M, Halpern BS, Jorge MA, Lombana A, Lourie SA, Martin KD, McManus E, Molnar J, Recchia CA, Robertson J (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 57:573–583
Stachowicz JJ, Fried H, Osman RW, Whitlach RB (2002) Biodiversity, invasion resistance, and marine ecosystem function: reconciling pattern and process. Ecology 83:2575–2590
Stefaniak L, Lambert G, Gittenberger A, Zhang H, Lin S, Whitlach RB (2009) Genetic conspecificity of the worldwide populations of Didemnum vexillum Kott, 2002. Aquat Invasions 4:29–44
Stimpson W (1852) Several new ascidians from the coast of the United States. Proc Boston Soc Nat Hist 4:228–238
Svane I, Young CM (1989) The ecology and behaviour of ascidian larvae. Oceanogr Mar Biol Annu Rev 27:45–90
Switzer SE, Therriault TW, Dunham A, Pearce CM (2011) Assessing potential control options for the invasive tunicate Didemnum vexillum in shellfish aquaculture. Aquaculture 318:145–153
Tracy B, Reyns N (2014) Spatial and temporal patterns of native and invasive ascidian assemblages in a southern California embayment. Aquat Invasions 9:441–455
Valentine PC, Collie JS, Reid RN, Asch RG, Guida VG, Blackwood DS (2007) The occurrence of the colonial ascidian Didemnum sp. on Georges Bank gravel habitat: ecological observations and potential effects on groundfish and scallop fisheries. J Exp Mar Biol Ecol 342:179–181
Van Name WG (1921) Ascidians of the West Indian region and southeastern United States. Bull Am Mus Nat Hist NY 44:283–494
Vandepas LE, Oliveira LM, Lee SSC, Hirose E, Rocha RM, Swalla BJ (2015) Biogeography of Phallusia nigra: is it really black and white? Biol Bull 228:52–64
Verling E, Ruiz GM, Smith D, Galil B, Miller AW, Murphy KR (2005) Supply-side invasion ecology: characterizing propagule pressure in coastal ecosystems. Proc R Soc Lond, Ser B: Biol Sci 272:1249–1257
Wasson K, Zabin CJ, Bedinger L, Diaz MC, Pearse JS (2001) Biological invasions of estuaries without international shipping: the importance of intraregional transport. Biol Conserv 102:143–153
Wasson K, Fenn K, Pearse JS (2005) Habitat differences in marine invasions of central California. Biol Invasions 7:935–946
Whitlatch RB, Osman RW (2000) Geographical distributions and organism-habitat associations of shallow-water introduced marine fauna in New England. In: Pederson J (ed). National Conference on Marine Bioinvasions, January 24–27, 1999., pp 61–65
Williams SL, Davidson IC, Pasari JR, Ashton GV, Carlton JT, Crafton RE, Fontana RE, Grosholz ED, Miller AW, Ruiz GM, Zabin CJ (2013) Managing multiple vectors for marine invasions in an increasingly connected world. Bioscience 63:952–966
Yund PO, Collins C, Johnson SL (2015) Evidence of a native northwest Atlantic COI haplotype clade in the cryptogenic colonial ascidian Botryllus schlosseri. Biol Bull 216:201–216
Zabin CJ, Ashton GV, Brown CW, Davidson IC, Sytsma MD, Ruiz GM (2014) Small boats provide connectivity for nonindigenous marine species between a highly invaded international port and nearby coastal harbors. Manag Biol Invasions 5:97–112
Zhan A, MacIsaac HJ, Cristescu ME (2010) Invasion genetics of the Ciona intestinalis species complex: from regional endemism to global homogeneity. Mol Ecol 19:4678–4694
Zhan A, Briski E, Bock DG, Ghabooli S, MacIsaac HJ (2015) Ascidians as models for studying invasion success. Mar Biol 162:2449–2470
Acknowledgments
This paper is dedicated to the late Charles C. Lambert, who encouraged and inspired many of us with his enthusiasm and knowledge of ascidians. We thank James T. Carlton and Rosana Rocha for expert advice on global ascidian distributions and Stacey Havard and Brian Steves for assisting with management and analysis of fouling plate survey data collected by the Smithsonian Marine Invasions Research Lab. We wish to thank the large number of lab members and volunteers who helped with fouling plate surveys, including: Scott Cowan, Esther Collinetti, Tami Huber and Linda McCann. This research was supported by funding from the California Department of Fish and Wildlife, National Sea Grant Program, Prince William Sound Regional Citizens’ Advisory Council, Smithsonian Institution, United States Coast Guard, US Fish and Wildlife Service, and the United States Department of Defense Legacy Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: E. Briski.
Reviewed by M. Carman and an undisclosed expert.
This article is part of the Topical Collection on Invasive Species.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00227-016-2995-7.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Simkanin, C., Fofonoff, P.W., Larson, K. et al. Spatial and temporal dynamics of ascidian invasions in the continental United States and Alaska. Mar Biol 163, 163 (2016). https://doi.org/10.1007/s00227-016-2924-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2924-9