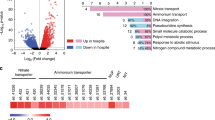
Abstract
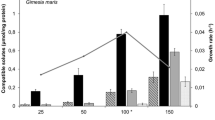
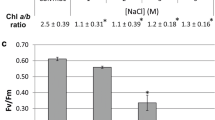
In marine nutrient-poor environments, the success of Symbiodinium–cnidarian symbioses depends on the translocation of photosynthetically fixed carbon to the host. However, the mechanisms involved in this translocation are not well understood. Glycerol has been identified in the literature as one of the main photosynthates translocated from symbiont to host. Considering that glycerol is a preferred regulatory osmolyte in many unicellular eukaryotes and exposure of isolated symbionts to host homogenate results in the liberation of glycerol, we investigated the potential role of glycerol and its release in Symbiodinium. We studied the response to high osmolarity in two cultured species of Symbiodinium, examining glycerol production, specific activity of the enzyme glycerol 3-phosphate dehydrogenase and expression of the coding gene. We also assessed the production of glycerol and glucose under osmotic stress and nitrogen depletion. Results showed that cells exposed to high osmolarity conditions induced the synthesis of glycerol, although it was not retained effectively inside cells. In addition, nitrogen depletion also induced the synthesis of glycerol, although values were an order of magnitude lower. Interestingly, a significant decrease in glucose levels was detected in osmotically stressed cultures and under low nitrogen, possibly associated with the storage of carbon. While glycerol is a metabolite induced by osmotic stress, our results do not support its role as osmolyte. We conclude that glycerol may have a role as a sink for reducing power to prevent feedback inhibition of photosynthesis, particularly under low nitrogen, conditions that may be prevalent in the symbiosis.






Similar content being viewed by others
References
Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250. doi:10.1158/0008-5472.CAN-04-0496
André L, Hemming A, Adler L (1991) Osmoregulation in Saccharomyces cerevisiae. Studies on the osmotic induction of glycerol production and glycerol 3-phosphate dehydrogenase (NAD+). FEBS Lett 286:13–17. doi:10.1016/0014-5793(91)80930-2
Bayer T, Aranda M, Sunagawa S, Yum LK, DeSalvo MK et al (2012) Symbiodinium transcriptomes: genome insights into the dinoflagellate symbionts of reef-building corals. PLoS One 7(4):e35269. doi:10.1371/journal.pone.0035269
Ben-Amotz A, Avron M (1983) Accumulation of metabolites by halotolerant algae and its industrial potential. Annu Rev Microbiol 37:95–119. doi:10.1146/annurev.mi.37.100183.000523
Bergmeyer J, Graβl M (1987) Methods of enzymatic analysis. Vol III. Enzymes I. Oxydorreductases, transferases. VCH Publishers, New York. ISSN: 3527260439
Blank RJ (1987) Cell architecture of the dinoflagellate Symbiodinium sp. inhabiting the Hawaiian stony coral Montastrea verrucosa. Mar Biol 94:143–155. doi:10.1007/BF00392906
Burch TA, Adams WW III, Degrenne BLS, Englert CH, Mines BR et al (2015) Environmental manipulation of growth and energy carrier release from freshwater and marine Chlamydomonas species. J Appl Phycol 27:1127–1136. doi:10.1007/s10811-014-0433-0
Burriesci MS, Raab TK, Pringle JR (2012) Evidence that glucose is the major transferred metabolite in dinoflagellate–cnidarian symbiosis. J Exp Biol 215:3467–3477. doi:10.1242/jeb.070946
Chapin FS (1991) Integrated responses of plants to stress. Bioscience 41:29–36. doi:10.2307/1311538
Chen H, Jiang J-G (2009) Osmotic responses of Dunaliella to the changes of salinity. J Cell Physiol 219:251–258. doi:10.1002/jcp.21715
Colombo-Pallota MF, Rodríguez-Román A, Iglesias-Prieto R (2010) Calcification in bleached and unbleached Montastraea faveolata: evaluating the role of oxygen and glycerol. Coral Reefs 29:899–907. doi:10.1007/s00338-010-0638-x
Cook CB, D’Elia CF (1987) Are natural populations of zooxanthellae ever nutrient limited? Symbiosis 4:199–211
Cook CB, D’Elia CF, Muller-Parker G (1988) Host feeding and nutrient sufficiency for zooxanthellae in the sea anemone Aiptasia pallida. Mar Biol 98:253–262. doi:10.1007/BF00391203
Cook CB, Muller-Parker G, Orlandini CD (1994) Ammonium enhancement of dark carbon fixation and nitrogen limitation in zooxanthellae symbiotic with the reef corals Madracis mirabilis and Montastrea annularis. Mar Biol 118:157–165. doi:10.1007/BF00699230
Dagenais BS, Dorion S, Rivoal J, Morse D (2014) The dinoflagellate Lingulodinium polyedrum responds to N depletion by a polarized deposition of starch and lipid bodies. PLoS One 9(11):e111067. doi:10.1371/journal.pone.0111067
Davy SK, Cook CB (2001a) The relationship between nutritional status and carbon flux in the zooxanthellate sea anemone Aiptasia pallida. Mar Biol 139:999–1005. doi:10.1007/s002270100640
Davy SK, Cook CB (2001b) The influence of ‘host release factor’ on carbon release by zooxanthellae isolated from fed and starved Aipatsia pallida (Verril). Comp Biochem Physiol Part A 129:487–494. doi:10.1016/S1095-6433(01)00285-9
Davy SK, Allemand D, Weis VM (2012) Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol Mol Rev 76:229–261. doi:10.1128/MMBR.05014-11
Dubinsky Z, Jokiel PL (1994) Ratios of energy and nutrient fluxes regulates symbiosis between zooxanthellae and corals. Pac Sci 48:313–324
Ezzat L, Magger JF, Grover R, Ferrier-Pagès C (2015) New insights into carbon acquisition and exchanges within the coral-dinoflagellate symbiosis under NH4 + and NO3 – supply. Proc R Soc B 282:20150610. doi:10.1098/rspb.2015.0610
Falkowski PG, Dubinzky Z, Muscatine L, McCloskey LR (1993) Population control in symbiotic corals. Bioscience 43:606–611. doi:10.2307/1312147
Ferrier-Pagès C, Gattuso JP, Dallot S, Jaubert J (2000) Effect of nutrient enrichment on growth and photosynthesis of the zooxanthellate coral Stylophora pistillata. Coral Reefs 19:103–113. doi:10.1007/s003380000078
Gancedo C, Gancedo JM, Sols A (1968) Glycerol metabolism in yeasts. Pathways of utilization and production. Eur J Biochem 5:165–172. doi:10.1111/j.1432-1033.1968.tb00353.x
Goiran C, Allemand D, Galgani I (1997) Transient Na+ stress in symbiotic dinoflagellates after isolation from coral-host cells and subsequent immersion in seawater. Mar Biol 129:581–589. doi:10.1007/s002270050199
Goyal A (2007) Osmoregulation in Dunaliella, part II: photosynthesis and starch contribute carbon for glycerol synthesis during a salt stress in Dunaliella tertiolecta. Plant Physiol Biochem 45:705–710. doi:10.1016/j.plaphy.2007.05.009
Grant AJ, Rémond M, People J, Hinde R (1997) Effects of host tissue homogenate of the scleractinian coral Plesiastrea versipora on glycerol metabolism in isolated symbiotic dinoflagellates. Mar Biol 128:665–670. doi:10.1007/s002270050133
Grant AJ, Rémond M, Hinde R (1998) Low molecular-weight factor from Plesiastrea versipora (Scleractinia) that modifies release and glicerol metabolism of isolated symbiotic algae. Mar Biol 130:553–557. doi:10.1007/s002270050276
Grant A, People J, Rémond M, Frankland S, Hinde R (2013) How a host cell signaling molecule modifies carbon metabolism in symbionts of the coral Plesiastrea versipora. FEBS J 280:2085–2096. doi:10.1111/febs.12233
Grover R, Maguer JF, Reynaud-Vaganay S, Ferrier-Pagès C (2002) Uptake of ammonium by the scleractinian coral Stylophora pistillata: effect of feeding, light, and ammonium concentrations. Limnol Oceanogr 47:782–790. doi:10.4319/lo.2002.47.3.0782
He Y, Meng X, Fan Q, Sun X, Xu Z et al (2009) Cloning and characterization of two novel chloroplastic glycerol 3-phosphate dehydrogenases from Dunaliella viridis. Plant Mol Biol 71:193–205. doi:10.1007/s11103-009-9517-7
Hoegh-Guldberg O, Hinde R (1986) Studies on a nudibranch that contains zooxanthellae I. Photosynthesis, respiration and the translocation of newly fixed carbon by zooxanthellae in Pteraeolidia ianthina. Proc R Soc Lond B 228:493–509. doi:10.1098/rspb.1986.0066
Holocomb M, Tambutté E, Allemand D, Tambutté S (2014) Light enhanced calcification in Stylophora pistillata: effects of glucose, glycerol and oxygen. PeerJ 2:e375. doi:10.7717/peerj.375
Islas-Flores T, Guillén G, Alvarado-Affantranger X, Lara-Flores M, Sánchez F, Villanueva MA (2011) PvRACK1 loss-of-function impairs cell expansion and morphogenesis in Phaseolus vulgaris L. root nodules. Mol Plant Microbe Interact 24:819–826. doi:10.1094/MPMI-11-10-0261
Jiang P-L, Pasaribu B, Chen C-S (2014) Nitrogen-deprivation elevates lipid levels in Symbiodinium spp. by lipid droplet accumulation: morphological and compositional analyses. PLoS One 9(1):e87416. doi:10.1371/journal.pone.0087416
Kremer BR (1980) Taxonomic implications of algal photoassimilate patterns. Br Phycol J 15:399–409
Kremer BP, Schmaljohann R, Röttger R (1980) Features and nutritional significance of photosynthates produced by unicellular algae symbiotic with larger foraminifera. Mar Ecol Prog Ser 2:225–228
LaJeunesse TC (2001) Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region: in search of a “species” level marker. J Phycol 37:866–880. doi:10.1046/j.1529-8817.2001.01031.x
LaJeunesse TC (2002) Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141:387–400. doi:10.1007/s00227-002-0829-2
Leggat W, Yellowlees D, Medina M (2011) Recent progress in Symbiodinium transcriptomics. J Exp Mar Biol Ecol 408:120–125. doi:10.1016/j.jembe.2011.07.032
León R, Galván F (1994) Halotolerance studies in Chlamydomonas reinhardtii: glycerol excretion by free and immobilized cells. J Appl Phycol 6:13–20. doi:10.1007/BF02185898
Lipschultz F, Cook C (2002) Uptake and assimilation of 15N-ammonium by the symbiotic sea anemones Bartholomea annulata and Aiptasia pallida: conservation versus recycling of nitrogen. Mar Biol 140:489–502. doi:10.1007/s00227-001-0717-1
Liska AJ, Shevchenko A, Pick U, Katz A (2004) Enhanced photosynthesis and redox energy production contribute to salinity tolerance in Dunaliella as revealed by homology-based proteomics. Plant Physiol 136:2806–2817. doi:10.1104/pp.104
Luo YJ, Wang LH, Chen WNU, Peng SE et al (2009) Ratiometric imaging of gastrodermal lipid bodies in coral-dinoflagellate endosymbiosis. Coral Reefs 28:289–301. doi:10.1007/s00338-008-0462-8
McGuire MP, Szmant AM (1997) Time course of physiological responses to NH4 enrichment by a coral-zooxanthellae symbiosis.In: Proc 8th Int Coral Reef Symp, vol 1. pp 909–914
Muller-Parker G, Lee K, Cook CB (1996) Changes in the ultrastructure of symbiotic zooxanthellae (Symbiodinium sp., Dinophyceae) in fed and starved sea anemones maintained under high light and low light. J Phycol 32:987–994. doi:10.1111/j.0022-3646.1996.00987.x
Muscatine L (1967) Glycerol excretion by symbiotic algae from corals and Tridacna and its control by the host. Science 156:516–519. doi:10.1126/science.156.3774.516
Muscatine L, Hand C (1958) Direct evidence for the transfer of materials from symbiotic algae to the tissues of a coelenterate. Proc Natl Acad Sci USA 44:1259–1263
Muscatine L, Porter JW (1977) Reefs corals: mutualistic symbioses adapted to nutrient poor environments. Bioscience 27:454–460. doi:10.2307/1297526
Muscatine L, Falkowski PG, Porter JW, Dubinski Z (1984) Fate of photosynthetic fixed carbon in light-adapted and shade-adapted colonies of the symbiotic coral Stylophora pistillata. Proc R Soc Lond B 222:181–202. doi:10.1098/rspb.1984.0058
Muscatine L, Falkowski PG, Dubinzky Z, Cook PA, McCloskey LR (1989) The effect of external nutrient resources on the population dynamics of zooxanthellae in a reef coral. Proc R Soc Lond B 236:311–324
Oren A (2005) A hundred years of Dunaliella research: 1905–2005. Saline Syst 1:1–14. doi:10.1186/1746-1448-1-2
Patton JS, Burris JE (1983) Lipid synthesis and extrusion by freshly isolated zooxanthellae (symbiotic algae). Mar Biol 75:131–136. doi:10.1007/BF00405995
Peng S-E, Chen C-S, Song Y-F, Huang H-T, Jiang P-L et al (2012) Assessment of metabolic modulation in free-living versus endosymbiotic Symbiodinium using synchrotron radiation-based infrared microspectroscopy. Biol Lett 8:434–437. doi:10.1098/rsbl.2011.0893
Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol Lett 26:509–515. doi:10.1023/B:BILE.0000019559.84305.47
Pochon X, Pawlowski J, Zaninetti L, Rowan R (2001) High genetic diversity and relative specificity among Symbiodinium-like endosymbiotic dinoflagellates in soritid foraminiferans. Mar Biol 139:1069–1078. doi:10.1007/s002270100674
Ritchie RJ, Grant AJ, Eltringham K, Hinde R (1997) Clotrimazole, a model compound for the host release factor of the coral Plesiastrea versipora. Funct Plant Biol 24:283–290. doi:10.1071/PP96106
Robledo D, Hernández-Urcera J, Cal RM, Pardo BG, Sánchez L, Martínez P, Viñas A (2014) Analysis of qPCR reference gene stability determination methods and a practical approach for efficiency calculation on a turbot (Scopthalmus maximus) gonad dataset. BMC Genom 15:648. doi:10.1186/1471-2164-15-648
Rodríguez-Román A, Iglesias-Prieto R (2005) Regulation of photochemical activity in cultured symbiotic dinoflagellates under nitrate limitation and deprivation. Mar Biol 146:1063–1073. doi:10.1007/s00227-004-1529-x
Rosic NN, Hoegh-Guldberg O (2010) A method for extracting high-quality RNA from Symbiodinium sp. J Appl Phycol 22:139–146. doi:10.1007/s10811-009-9433-x
Rosic NN, Pernice M, Dove S, Dunn S, Hoegh-Guldberg O (2011) Gene expression profiles of cytosolic heat shock proteins Hsp70 and Hsp90 from symbiotic dinoflagellates in response to thermal stress: possible implications for coral bleaching. Cell Stress Chaperones 16:69–80. doi:10.1007/s12192-010-0222-x
Rowan R (1998) Review—diversity and ecology of zooxanthellae on coral reefs. J Phyol 34:407–417. doi:10.1046/j.1529-8817.1998.340407.x
Schmitz K, Kremer BP (1977) Carbon fixation and analysis of assimilates in a coral-dinoflagellate symbiosis. Mar Biol 42:305–313. doi:10.1007/BF00402192
Seibt C, Schlichter D (2001) Compatible intracellular ion composition of the host improves carbon assimilation by zooxanthellae in mutualistic symbioses. Naturwissenschaften 88:382–386. doi:10.1007/s001140100240
Silver N, Best S, Jiang J, Thein SL (2006) Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol 7:33. doi:10.1186/1471-2199-7-33
Sols A, Gancedo C, de la Fuente G (1971) Energy-yielding metabolism in yeasts. In: Rose AH (ed) The yeasts. Academic Press, London, pp 271–307
Suescún-Bolívar LP, Thomé PE (2015) Osmosensing and osmoregulation in unicellular eukaryotes. World J Microbiol Biotechnol 31:435–443. doi:10.1007/s11274-015-1811-8
Suescún-Bolívar LP, Iglesias-Prieto R, Thomé PE (2012) Induction of glycerol synthesis and release in cultured Symbiodinium. PLoS One 7(10):e47182. doi:10.1371/journal.pone.0047182
Sutton DC, Hoegh-Guldberg O (1990) Host-zooxanthellae interactions in four temperate marine invertebrate symbioses: assessment of effect of host extracts on symbionts. Biol Bull 178:175–186
Swanson R, Hoegh-Guldberg O (1998) Amino acid synthesis in the symbiotic sea anemone Aiptasia pulchella. Mar Biol 131:83–93. doi:10.1007/s002270050299
Takeuchi T, Endo K (2006) Biphasic and dual coordinated expression of the genes encoding major shell matrix proteins in the pearl oyster Pinctada fucata. Mar Biotechnol 8:52–61. doi:10.1007/s10126-005-5037-x
Tanaka Y, Miyajima T, Koike I, Hayashibara T, Ogawa H (2006) Translocation and conservation of organic nitrogen within the coral-zooxanthella symbiotic system of Acropora pulchra, as demonstrated by dual isotope-labeling techniques. J Exp Mar Biol Ecol 336:110–119. doi:10.1016/j.jembe.2006.04.011
Tremblay P, Grover R, Maguer JF, Legendre L, Ferrier-Pagès C (2012) Autotrophic carbon budget in coral tissue: a new 13C-based model of photosynthate translocation. J Exp Biol 215:1384–1393. doi:10.1242/jeb.065201
Trench RK (1971) The physiology and biochemistry of zooxanthellae symbiotic with marine coelenterates. II. Liberation of fixed 14C by zooxanthellae in vitro. Proc R Soc Lond B 177:237–250. doi:10.1098/rspb.1971.0025
Trench RK (1979) The cell biology of plant-animal symbiosis. Annu Rev Plant Physiol 30:485–531. doi:10.1146/annurev.pp.30.060179.002413
Valdesompele J, De Peter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:research0034.1. doi:10.1186/gb-2002-3-7-research0034
Wang JT, Douglas AE (1997) Nutrients, signals, and photosynthate release by symbiotic algae (the impact of taurine on the dinoflagellate alga Symbiodinium from the sea anemone Aiptasia pulchella). Plant Physiol 114:631–636. doi:10.1104/pp.114.2.631
Whitehead LF, Douglas AE (2003) Metabolite comparisons and the identity of nutrients translocated from symbiotic algae to an animal host. J Exp Biol 206:3149–3157. doi:10.1242/jeb.00539
Yancey PH, Heppenstall M, Ly S, Andrell RM, Gates RD et al (2010) Betaines and dimethylsulfoniopropionate as major osmolytes in cnidaria with endosymbiotic dinoflagellates. Physiol Biochem Zool 83:167–173. doi:10.1086/644625
Yellowlees D, Rees TA, Leggat W (2008) Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ 31:679–694. doi:10.1111/j.1365-3040.2008.01802
Acknowledgments
LPS-B and GMIT acknowledge a graduate fellowship from the Consejo Nacional de Ciencia y Tecnología, México. We thank Dr. Iglesias-Prieto and three anonymous reviewers for improving the manuscript, Luis F. Ortiz-Matamoros for the cloning of the GPD fragment, and Elisa López for retyping the Symbiodinium cultures employed in this work. This work was partially financed by UNAM-DGAPA-PAPIIT Grant Number IN205714 to PET.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: K. Bischof.
Reviewed by Undisclosed experts
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suescún-Bolívar, L.P., Traverse, G.M.I. & Thomé, P.E. Glycerol outflow in Symbiodinium under osmotic and nitrogen stress. Mar Biol 163, 128 (2016). https://doi.org/10.1007/s00227-016-2899-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2899-6