Abstract
Many wildlife studies use chemical analyses to explore spatio-temporal variation in diet, migratory patterns and contaminant exposure. Intrinsic markers are particularly valuable for studying non-breeding marine predators, when direct methods of investigation are rarely feasible. However, any inferences regarding foraging ecology are dependent upon the time scale over which tissues such as feathers are formed. In this study, we validate the use of body feathers for studying non-breeding foraging patterns in a pelagic seabird, the northern fulmar. Analysis of carcasses of successfully breeding adult fulmars indicated that body feathers moulted between September and March, whereas analyses of carcasses and activity patterns suggested that wing feather and tail feather moult occurred during more restricted periods (September to October and September to January, respectively). By randomly sampling relevant body feathers, average values for individual birds were shown to be consistent. We also integrated chemical analyses of body feather with geolocation tracking data to demonstrate that analyses of δ13C and δ15N values successfully assigned 88 % of birds to one of two broad wintering regions used by breeding adult fulmars from a Scottish study colony. These data provide strong support for the use of body feathers as a tool for exploring non-breeding foraging patterns and diet in wide-ranging, pelagic seabirds.
Similar content being viewed by others
Introduction
Marine organisms are exposed to a variety of naturally occurring and anthropogenic chemicals. These have the potential to provide biogeochemical markers that can underpin studies of both spatial and foraging ecology (Ramos and González-Solís 2012). Wide-ranging marine top predators are likely to experience large spatial variation in the distribution of chemical tracers (Burger and Gochfield 2000; Gómez-Díaz and González-Solís 2007; Hobson and Bond 2012). Previous studies have used these markers to investigate contaminant levels (Bond and Lavers 2011; Moreno et al. 2011), diet (Hooker et al. 2001; Fisk et al. 2002) and migratory patterns (Hobson, 1999; Seminoff et al. 2012). However, detailed interpretation of these results ideally requires information on both where and when those chemicals were incorporated into the target tissues (Burger and Gochfeld 2000; Polizzi et al. 2013).
Analysis of intrinsic markers is particularly suited for seabirds, which can be sampled when they return annually to their breeding colonies (Furness and Camphuysen 1997). The different tissue types that may be sampled (e.g., feather, blood, muscle) represent different timescales over which the chemicals have been assimilated (Pearson et al. 2003; Bearhop et al. 2004). However, the distribution of birds breeding at particular colonies during the preceding winter is often uncertain. This is of particular importance when making colony-level inferences about foraging, because birds breeding at the same colony may exhibit individual differences in wintering areas (Kubetzki et al. 2009; Harris et al. 2010; Kopp et al. 2011). Thus, whilst stable isotopes have been commonly used to study winter foraging ecology (Cherel et al. 2006; Dehnhard et al. 2011), few studies have been based upon individuals with known foraging patterns (cf. Furness et al. 2006; Phillips et al. 2009; Jaeger et al. 2010; Leat et al. 2013). Recent advances in tracking technology now provide opportunities to validate these chemical-based methodologies for a wider range of species.
Feathers are generally the preferred tissue for studies of this kind because they are metabolically inert (Inger and Bearhop 2008; Hobson and Bond 2012) and relatively easily collected from breeding individuals (Bearhop et al. 2002). However, this requires information on the study species’ moult pattern to relate chemical results to a particular time period or location (Inger and Bearhop 2008). Whilst the pattern of moult in different feathers is well understood in many terrestrial species (Hinsley et al. 2003; Ryder and Wolfe 2009; Gargallo 2013), comparable data are rare from seabirds because they spend so much of the year at sea. Nevertheless, whilst moult in temperate and polar seabirds may commence towards the end of a reproductive attempt, most feather formation occurs within the non-breeding period (Quillfeldt et al. 2005; Bridge 2006; Allard et al. 2008). Most data for seabirds relate to the timing of moult in primary and secondary wing feathers (Ginn and Melville 1983) or for species with a unique, simultaneous body moult pattern (Carravieri et al. 2014). However, it is not possible to take whole wing feathers from live birds, and sub-samples of feather tips (Cherel et al. 2000) relate to relatively short periods of growth. Alternatively, chemical analyses of body feathers may provide a more general indication of foraging activity through the non-breeding period (Bearhop et al. 2006). However, for many species, limited understanding of the timing of moult and variability in the chemical composition of different body feathers constrains the extent to which chemical signatures can be used to explore winter foraging patterns (Larson and Hobson 2009).
Recent tracking data identified marked individual differences in wintering location within a North Atlantic colony of northern fulmars (hereafter fulmar, Fulmarus glacialis) (Quinn 2014). Furthermore, individual birds were distributed widely but tended to be consistent in their use of two broad-scale over-wintering areas (deep Atlantic waters, and relatively shallow waters over the continental shelf) that are likely to differ in their biogeochemistry. This study system provides a rare opportunity to integrate analyses of feather chemistry with seabird tracking data on winter distribution, to test for spatial differences in intrinsic markers. However, additional work is first required to assess whether chemical composition of body feathers is representative of the winter non-breeding period. Existing information on moult patterns in Procellariformes such as fulmars is limited, and based either on observations of birds at breeding colonies (Carrick and Dunnet 1954; Allard et al. 2008) or analysis of beached or by-caught individuals during the non-breeding season (van Franeker 2004). These limited data suggest that, in fulmars, adult moult begins post-breeding (late August) (Carrick and Dunnet 1954; Allard et al. 2008) and is be completed by the end of February (Ginn and Melville 1983).
This study aimed to validate the use of body feathers, specifically those which are easily collected from the ventral region, to assess individual and spatial variation in trace metals and stable isotope markers over the non-breeding period. If there is co-variation in chemical composition in wing feathers and body feathers, it would suggest growth of these different feathers occurs over a similar period. In contrast, if body feathers grow later in the winter, they may be more similar in composition to tail feathers, as fulmars are known to initiate tail moult when wing moult is around 75 % complete (Ginn and Melville 1983).
Using a combination of morphological, tracking and chemical analyses, the specific objectives were to: (a) assess seasonal variation in activity patterns to identify most likely period of moult, (b) compare moult timings and chemical loadings in wing, tail and body feathers, (c) assess within-individual variability in chemical loadings in body feathers, and (d) to validate the use of body feathers as a proxy for identifying broad scale wintering areas.
Materials and methods
Study site and logger deployment
Data on winter distribution, activity and feather chemistry were collected from adult fulmars breeding on Eynhallow, Orkney (59°8′N; 3°8′W). Fieldwork was conducted between 2006 and 2012 in parallel with ongoing population studies (see Lewis et al. 2009; Cordes et al. 2015), when approximately 100 active nests were recorded annually. Breeding adults were caught at the nest using a hand net or noose under licence from the British Trust for Ornithology, and British Antarctic Survey (BAS) light-based Global Location Sensor (GLS) loggers (Phillips et al. 2004) were attached to the darvic leg-rings of 163 birds using cable ties. The total device weight (including leg ring) was 3.6 g, representing <0.5 % of the lightest recorded fulmar’s body weight. Annual attendance of breeding adults in this study population varies with environmental conditions and may be as low as 50 % in some years (Thompson and Ollason 2001). This can constrain and delay logger recovery rates, which were 46 % over one year and 76 % over two or more years. After re-capture, a randomly selected sample of six to ten body feathers were taken from the body region of tagged individuals under licence from the UK Home Office. Data on trace metals in feathers collected from birds at the end of the winter in which they had been tracked were available from 46 birds. Concurrent data on C and N isotope data were available from 37 birds. Sex was determined by molecular analysis of DNA from feathers collected during logger recovery, using P2-P8 primer sequences and Z-002 and CAM-11 markers (Griffiths et al. 1998; Dawson 2008).
Seasonal variation in activity patterns
Wet-dry sensors in the GLS loggers recorded whether tags were wet or dry every 3 s (see Mackley et al. 2011). Summary data on the number of wet samples in each 10 min period were then stored, allowing each 10 min period to be classified as wet, dry or mixed. These data were then used to explore seasonal patterns in the mean proportion of time spent dry in each month, to provide an indication of when flight performance may be constrained by the moult. Activity data were available for 68 individuals [post-breeding season (Sept–Dec): 33 females, 35 males, complete non-breeding season (Sept–May): 26 females, 29 males].
The timing of feather moult
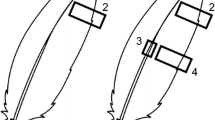
Data on feather moult were collected from carcasses during a long-term study of plastic contamination (van Franeker et al. 2011). Full details of the techniques used can be found in the Fulmar Litter EcoQO Manual Part 1: Collection and dissection procedures (van Franeker 2004). Primary and tail moult scores were recorded for each bird using criteria developed by the British Trust for Ornithology (BTO) (Ginn and Melville 1983). Moult scores were based upon external inspection of the feather, where an old feather was scored as 0 and a new, fully developed feather as 5. The maximum primary moult score for a fulmar was therefore 100, as fulmars have a total of 20 primary feathers. The maximum tail moult score was 70, as fulmars normally have 14 tail feathers. For the purposes of this study, April 1st was used as the reset date for the completion of moult, after which new plumage (total score 100 or score 70) must become old plumage (score 0) before the next moult cycle. Body feather moult was scored by internal assessment of the degree of active moult in body feathers in the sternal region of the ventral feather tract. Actively moulting feathers were identified by the broad and soft whitish (sometimes somewhat bluish) feather shafts, differing from the sturdy and pointed pins of full grown feathers (see Supplementary material Fig. 1). Moult was then scored as: 0 = no new feather shafts present in left or right sternal feather tract; 1 = 1–4 new soft whitish feather shafts in either or each of left or right sternal feather tract; and 2 = 5 or more new feather shafts present in either or each of left or right sternal feather tract section. Data were available from 725 adult fulmars which were defined as breeders based upon internal examination (see Supplementary material Table 1 for month by month sample sizes). These birds were either by-caught on Faroese or Icelandic long-lines, or recovered from local harvests in these areas, but were otherwise recorded as being in apparently healthy condition based upon body condition scores during dissection (van Franeker 2004). Mean moult scores for each feather type were calculated for each month of the year. These were then used to estimate the proportion of birds (with associated standard errors) that had completed moult (primary and tail, feathers) or were actively moulting body feathers, in each month.
It is assumed for the purposes of this study that moult data from the adult birds caught off the Faroes and Iceland are representative of the moult pattern at North Sea colonies, including our main study site in Orkney.
Chemical analyses of feathers
All feathers were first washed with Milli-Q water prior to oven drying at 65 °C for >12 h. For the trace metal analysis, aristar nitric acid (0.5 ml) was added to each feather sample and left overnight to digest. Aristar hydrogen peroxide (1 ml) was then added, before a 75 min microwave digest (CEM Microwave Technology Ltd.) in which a peak temperature of 95 °C was held for 30 min. Samples were then diluted to 10 ml with ultrapure deionized Milli-Q water, and 19 element total concentrations were measured from a sub-sample using an ICP-MS 7500 (Agilent Technologies), using the reaction cell with hydrogen gas. Bovine liver (National Institute of Standards and Technology) 1557b was used as the certified reference material (CRM). Standards made from multi-element solution 2 were run every 30 samples, from which standard curves could be calculated. In a few samples, some elements (V, Mn, As, Cd, Pb) were below the limits of detection (LoD). Following standard procedure (Anderson et al. 2010), these samples were given a value of half the LoD. All trace metal analyses were conducted at the University of Aberdeen.
For C and N isotope analyses, dried samples were homogenized in a ball mill (Mixer Mill type MM 200, Retsch of Haan, Germany) and sub-samples of 1.3 mg material loaded into 5 × 3.5 mm tin cups (Elemental Microanalysis Ltd.). Total N and C values and the 15N:14N and 13C:12C isotope ratios of milled dried material were determined using a Flash EA 1112 Series Elemental Analyser connected via a Conflo III to a DeltaPlus XP isotope ratio mass spectrometer (all Thermo Finnigan, Bremen, Germany). Isotope ratios were calculated using CO2 and N2 reference gasses injected with every sample. The isotopic values of these gasses were directly referenced against IAEA reference materials USGS40 and USGS41 (both l-glutamic acid); certified both for δ13C (‰VPDB) and δ15N (‰air N2). Long-term precision of a quality control standard (milled flour) was: δ13C 25.5 ± 0.29 ‰ and 15N 0.367 ± 0.0002 atom % (mean ± SD, n = 200). Samples of feathers from ten birds that were by-caught on Faroese long-lines were used to assess within-individual variation in chemical composition using four different types of feathers: primary (inners), secondary (opposite primaries), tail (central rectrices) and body (selection) feathers. Average sample weights were: primary (0.043 ± 0.009), secondary (0.040 ± 0.006), tail (0.051 ± 0007) and body (0.025 ± 0.003). Data were available for associated trace metals for between-feather comparisons, but not for C and N isotopic data. All stable isotope analyses were conducted at the James Hutton Institute.
Median concentrations of all elements above the limits of detection (LoD) were calculated for each feather type. Due to unequal variances between feather types, data were ranked (Langin et al. 2007) before performing repeated-measure ANOVAS. Feather type was the explanatory variable in the ANOVA, with each metal (loid) tested separately as the response variable. Individual bird ID was included as an error term to account for non-independence of individuals with repeated measures of feather types. Model fits were verified and pair-wise comparisons were made between each feather type using Tukey’s honest significant difference tests.
Between-feather variability in the chemical composition of body feathers was assessed using independent analysis of four or five feathers from ten individual birds that were sampled from the Eynhallow breeding population. For comparison with individuals in which measurements were based solely upon analyses of two body feathers, two body feathers were randomly sampled from each of these ten birds and the mean value calculated for each element (mg/kg). This process was then repeated for another two body feathers, and the two means were compared using the Pearson’s product moment correlation coefficient.
Identification of non-breeding areas
For the sample of Eynhallow breeders that were successfully instrumented with GLS loggers, twice-daily light-based geolocations for each individual were produced using BASTrak software v.18. A light threshold of 10 and an elevation angle of −3.5 were used, prior to an iterative smoothing process that was applied twice to the data to reduce location errors (Phillips et al. 2004).
A mean location was then calculated for each of these individuals over the entire winter period between the equinoxes (October–February) and projected in ArcGIS v.9.3 using the North Pole Lambert Azimuthal Equal Area projection. These data were then used to assign each individual bird to one of the two broad-scale wintering regions (oceanic Atlantic waters and more nearshore waters over the continental shelf). If data from an individual bird were available from multiple years, only 1 year was randomly chosen for analysis to maintain independence.
For the 46 birds with both GLS tracking data and data on trace metals in body feathers, a MANOVA was carried out on all elements against location group to assess for differences in trace metal (loid)s between location groups.
For the 37 birds with a full suite of feather chemistry data, we then explored whether the trace metal and isotopic signatures of body feathers collected upon recapture could be used to identify the wintering region that each of these individuals had used. A linear discriminant analysis (lda) was carried out in the ‘MASS’ package in R (Venables and Ripley 2002) using trace element and stable isotope data from body feathers. Cross-validation techniques were employed to produce jackknifed predictions (Leat et al. 2013) which assigned birds to one of the two broad wintering regions with a given probability level. Initially, the discriminant analysis was run using data for each of the trace elements and both δ13C and δ15N stable isotope data. Based upon exploration of levels of variation between individual using the two wintering regions, these results were then compared with alternative models that used only C and N stable isotope ratios and finally, only N stable isotope ratios.
All statistical analyses were performed in R v.2.15.0 (R Development Core Team 2012), and statistical significance was taken to be p < 0.05.
Results
Seasonal variation in activity patterns
Activity data from GLS loggers demonstrated that September to December were the months in which individual birds spent the highest proportion of their time on the water (Fig. 1). Marked seasonal variation in individual activity patterns was seen in both males and females (Fig. 1), but there were slight differences between the sexes in April and May co-incident with the pre-laying exodus and early incubation period. Both sexes spent the highest proportion of time on the water in September (90.5 and 91.5 % wet for females and males, respectively) and the least amount of time wet in June (29.1 and 29 % dry for females and males, respectively).
Variability in moult timings and chemical concentrations in different feather types
Analysis of moult scores revealed that primary moult in healthy adults occurred during September and October and was complete by November (Fig. 2). Little overlap with the breeding period in either wing or tail moult was recorded: the few initial phases of primary moult that were observed from June to August, were considered to represent an early start of moult amongst adults after breeding failure. Active tail moult in adults was seen over a longer period, from September to January, and was completed by February. Internal body moult was observed from September onwards, with the highest levels of active moult recorded in birds caught in October and December. However, these data indicate that active body moult occurs in adults throughout the non-breeding period (September to March) (Fig. 2).
Variation in the proportion of adult fulmars sampled in each month that had a completed their primary moult (n = 704) and b completed their tail moult (n = 703). The arrow represents the reset date on April 1st. c Monthly variation in body moult activity, derived from the average monthly score for internal body moult expressed as a percentage of its maximum score of 2 (n = 725). Data are based upon analysis of apparently healthy harvested and by-caught birds from Faroese and Icelandic waters. Sample sizes are provided in Supplementary Table 1, and average moult scores for each feather type are provided in Supplementary Table 2
Instrumental quality results for the chemical analyses demonstrated high percentage recovery for each element (Supplementary Table 3). In general, body feathers demonstrated smaller ranges in element concentrations than primary, secondary and tail feathers (Table 1). Fe and Zn, two essential elements, had the highest concentrations of all the elements studied and demonstrated large ranges (Table 1). All elements except As demonstrated a high degree of variability between feather types. Results of statistical comparisons of differences between the feather types revealed tail feathers had more significantly different pair-wise comparisons than any other feather type (9 of 13 elemental comparisons) and that primary and secondary feathers were similar in all but one element (V). Body feathers had similar metal (loid) concentrations to primary and secondary feathers in all but three elements (Supplementary Table 4). Tail feathers tended to have highest median concentrations of all elements compared to body, primary and secondary feathers, except for As, Se and Sr.
Coefficients of variation were sometimes high when multiple body feathers were measured from the same bird (Table 2). However, variation between individuals was generally much greater. Thus, when the means of two randomly selected pairs of feathers from the same individual were compared, there was a positive, significant relationship between repeat measurements (Table 2).
Identification of non-breeding areas from intrinsic markers in feathers
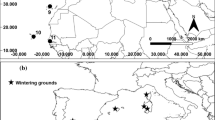
The sample of birds from which both GLS tracking and feather chemistry data were available included individuals that wintered over both Atlantic and continental shelf waters. The mean locations of each of these birds through the overall winter period (October–February) are shown in Fig. 3, together with the region that each bird was assigned to.
Mean winter location for the GLS tagged breeding fulmars for which data were also available on body feather chemical signatures. Mean winter locations are based upon daily locations obtained for each individual between mid-October to end of February. Based upon these mean locations, individuals were assigned to one of two broad wintering regions (Oceanic birds grey, Continental shelf birds black). The location of the Eynhallow breeding colony location is marked as a star
When considering each element separately, concentrations were similar between the group of birds wintering over the Atlantic and the group wintering over the continental shelf (Supplementary Table 5). Furthermore, there were no significant differences between groups when all elements were considered together (MANOVA, Pillai = 0.29, F (9,36) = 1.61, Pr(>F) = 0.151).
There were also no significant differences in δ13C between location groups (ANOVA, F (1,36) = 0.21, p = 0.649) but δ15N values were significantly different for birds wintering over the Atlantic and those wintering over the continental shelf (ANOVA, F (1,36) = 53.11, p < 0.001) (Table 3).
Using C and N isotope data, the discriminant analysis was able to correctly assign a high proportion (88 %) of birds to these different wintering regions (Table 4). Using the trace element data either alone or in combination with the isotope data resulted in lower predictive value (Supplementary Table 6). Raising the threshold for positive assignment reduced the number of individuals that could be assigned, but reduced the number of errors (Fig. 4). A cut off of 0.9 resulted in 100 % correctly assigned, but only 59 % of individuals could be assigned to a wintering region. Using a cut off of 0.8, 80 % of individuals were assigned to a wintering region, with a success rate of >95 %.
Variation in the percentage of birds that could be assigned to one of the two wintering regions from their feather isotope ratios (filled square) and the percentage of those assignments that were correct (filled triangle) in relation to the threshold probability used in the linear discriminant analysis
Discussion
This study has demonstrated that the chemical composition of body feathers can be used to make inferences about winter foraging ecology. Analysis of data from post-mortem investigations confirmed the time period over which body feathers grow in this species, thereby validating their use in diet and distribution studies over this period. Despite high inter-feather variability, mean values from pairs of body feathers provided a robust measure of individual variation in chemical loadings. These analyses, used in combination with tracking data from a sub-set of individuals, indicate that stable isotope markers in body feathers can provide insights into individual variability in the winter distribution patterns of breeding fulmars.
Timing of moult
Activity data collected from breeding adults indicated that flight time was reduced during September and October (Fig. 1), supporting previous suggestions that flight feathers are moulted at this time of year (Dott 1973). These findings build upon recent work on Antarctic procellarids (Cherel et al. 2016) that highlighted the value of using activity data from GLS loggers to identify the timing of moult. The pelagic nature of this species has compromised efforts to confirm when and where adult fulmars from particular breeding colonies moult. Direct observations of feather moult have been restricted either to fulmars harvested or by-caught in the northern part of their range, or beached individuals that are more commonly recovered from the south of their range. Tracking data collected during this study highlight the extensive winter ranges of birds from our primary study colony (Fig. 3) and provide confidence in our assumption that data on moult patterns of healthy adults of breeding age that were harvested or by-caught in Icelandic and Faroese waters are representative of birds from UK colonies.
The direct observations of moult in this sample of fulmars indicate that body feathers moult over a longer time span than primary feathers (Fig. 2), supporting their use as a tool for assessing trophic patterns over the non-breeding period. A shorter period of primary moult post-breeding, in conjunction with a more protracted period of body feather moult, is seen in other similar-sized seabirds (Furness et al. 1986; Bridge 2006). Wing moult constrains flight ability (Hedenström and Sunada 1999; Bridge 2006) and minimizing this period may be required to increase foraging opportunities at this critical time of year. In contrast, a longer period of body moult should not impede flight capabilities. Our data revealed no overlap between the breeding period (April to August) and either wing or tail moult initiation, confirming results from studies of fulmars breeding in the Canadian High Arctic (Allard et al. 2008). Similar patterns are found in other terrestrial and marine birds (Weimerskirch 1991; Hemborg et al. 2001), though some exceptions in the extent of overlap between breeding and moult do exist (see Barbraud and Chastel 1998; Ramos et al. 2009).
Chemical concentrations in body feathers were similar to those found in primary feathers (Table 1), suggesting the feathers were grown under similar conditions with respect to time, place or dietary intake. This concurs with other studies which demonstrated similarities in certain isotope levels between primary and breast feathers in wandering albatrosses Diomedea exulans (Jaeger et al. 2009) and common guillemots Uria aalge (Becker et al. 2007). In contrast, there were some differences between tail feathers and the other feather types, with tail feathers having higher concentrations of most elements. In fulmars, tail moult occurred in the latter half of winter, but this pattern of tail moult occurring after wing moult is not ubiquitous amongst other petrel species (see Hedd and Montevecchi 2006). Observed differences in chemical loadings in tail feathers could result from biological factors, such as differences in moult pattern or area; or fundamental differences in the sequestering of elements in tail feathers. Alternatively, differences may result from methodological issues related to feather preparation and external contamination of tail feathers. Tail feathers have a more complex structure than body feathers, and it is possible that, despite the Milli-Q water washing stage, external dust may have remained embedded in the feather (Font et al. 2007). External contamination of certain elements, commonly Fe, Hg, Ni and Zn, has been suggested in other feather studies (Dauwe et al. 2003) and the presence of heavy metals in preen oil (Goede and De Bruin 1984; Jaspers et al. 2008) may have affected chemical loadings in tail feathers. Whatever the cause of these differences, these data suggest that more detailed comparison of feather chemistry should avoid using tail feathers.
Within-individual variability in the chemical composition of body feathers
Due to their less complex structure, the chemical loadings of body feathers may be less likely to be influenced by external contamination. Whole body feathers can also be collected non-intrusively during routine handling of live birds, and they have been used as a tissue of choice in a wide variety of seabird studies (Bearhop et al. 2000; Cherel et al. 2006). However, in contrast to studies where specific regions of a clearly defined wing or tail feather are sampled (Barrett et al. 2007; Kouwenberg et al. 2013), analyses are typically based upon a small number of randomly sampled body feathers. Interpretation of between-individual differences in chemical signatures from these samples must therefore be placed in the context of within-individual variability. Existing work on within-individual variation in chemical loadings has generally compared different feather types (Thompson and Furness 1995; Deben et al. 2012), but two studies have previously focused on variation within body feathers. Jaeger et al. (2009) considered the advantages and disadvantages of using an averaged value for a pooled sample of body feathers compared to average values from feathers subjected to independent analysis, whilst Carravieri et al. (2014) compared individual variation in body feathers from seabirds with synchronous and asynchronous moult patterns. As seen by Carravieri et al. (2014) in other seabirds with a more protracted moult, our analyses also found a high level of within-individual variation in levels of some trace metals in fulmar body feathers. For example, Fe varied markedly between feathers, as shown in other seabird studies (Jerez et al. 2011). Nevertheless, there were strong positive correlations in the overall trace metal signatures of paired independent samples of body feathers from the same individual, supporting further use of this easily sampled tissue in biogeochemical studies of northern fulmars.
As reported in previous studies of large procellarids (Phillips et al. 2009) and Great skuas Stercorarius skua (Leat et al. 2013), our integration of feather chemistry and tracking data indicated that C and N isotopes in body feathers provide a good indication of each individual’s wintering region (Table 4). Generally, it has been C isotopes in feathers that have been most informative given the underlying geographical variation in C isotopes in the marine environment (Cherel and Hobson 2007). However, in our study, all the discriminatory power came from the variation in N isotopes and there was no difference in C isotopes between birds wintering in different regions. Consequently, observed geographical variation in these fulmars could result from differences in the foraging strategies of birds using different regions, with those individuals occurring over oceanic waters foraging at lower trophic levels (Table 3). Alternatively, δ15N values at the base of the food chain may differ in these different regions. Further work is now required to discriminate between these alternatives, as this has important implications for understanding the nature of the variation in non-breeding strategies observed in this species.
Using δ15N values either alone or in combination with δ13C, 88 % of birds were correctly assigned, whereas using additional information from the trace metal analysis decreased our ability to predict wintering regions. However, it remains important to explore the value of a broad suite of markers when applying this approach in different systems. For example, Gómez-Díaz and González-Solís (2007) found that trace metals gave greater discriminatory power than stable isotopes when assigning other Procellariformes to different Atlantic and Mediterranean colonies. In our study, restricting the number of birds assigned by introducing a threshold for the bootstrap probability resulted in lower errors (Fig. 4), and future studies using this approach should carefully balance the need to maximize the proportion of birds assigned with the level of confidence in those assignments.
Implications for future work
Intrinsic biogeochemical markers and tracking technologies provide increasing potential for studies of migratory patterns and foraging ecology. The integration of these techniques can be especially valuable, providing direct estimates of distribution and indirect information on diet and contaminant exposure in those areas. Body feathers provide an easily sampled tissue that can underpin these studies, but data interpretation can be constrained in many seabirds where there is limited information on moult patterns (Bridge 2006). Our study highlights that body feathers from fulmars can act as robust proxies for identifying individual variation in location and dietary patterns during the non-breeding period. Carefully designed feather sampling regimes can therefore provide a non-lethal sampling technique to improve our knowledge of seabird foraging during the non-breeding period. Some of these opportunities will be realized through biogeochemical studies that are integrated into the growing number of seabird tracking studies. It would be especially valuable to extend the study to other North Atlantic and North Pacific fulmar colonies to explore the extent to which this approach can be used in other regions. Other opportunities could result from the availability of much larger samples of feather samples compared to most tracking studies, and the availability of both contemporary and historic samples (e.g., Thompson et al. 1995). One important consideration is that study individuals need only be captured once to obtain a feather sample, whereas most tracking studies require a re-capture to retrieve a data logger (Hobson and Norris 2008). Recent tracking studies of a variety of seabird species have revealed that there is often within colony variation in wintering strategies, but the extent to which these may influence other aspects of behaviour that could bias tag retrieval remains uncertain. Our confirmation that body feather isotopic signatures reflect different wintering strategies at this UK colony now provide an opportunity to evaluate this bias, and further explore the nature of these alternative strategies and their population consequences.
References
Allard KA, Mallory ML, Wilcox KL, Forbes MR (2008) Prebasic molt initiation and progress in northern fulmars of the High Arctic: do molt and breeding overlap? Polar Biol 31:181–188. doi:10.1007/s00300-007-0345-4
Anderson ORJ, Phillips RA, Shore RF, McGill RAR, McDonald RA, Bearhop S (2010) Element patterns in albatrosses and petrels: influence of trophic position, foraging range, and prey type. Environ Pollut 158:98–107. doi:10.1016/j.envpol.2009.07.040
Barbraud C, Chastel O (1998) Southern fulmars molt their primary feathers while incubating. Condor 100:563–566. doi:10.2307/1369726
Barrett RT et al (2007) Diet studies of seabirds: a review and recommendations. ICES J Mar Sci 64:1675–1691. doi:10.1093/icesjms/fsm152
Bearhop S, Phillips RA, Thompson DR, Waldron S, Furness RW (2000) Variability in mercury concentrations of great skuas Catharacta skua: the influence of colony, diet and trophic status inferred from stable isotope signatures. Mar Ecol Prog Ser 195:261–268. doi:10.3354/meps195261
Bearhop S, Waldron S, Votier SC, Furness RW (2002) Factors that influence assimilation rates and fractionation of nitrogen and carbon stable isotopes in avian blood and feathers. Physiol Biochem Zool 75:451–458. doi:10.1086/342800
Bearhop S, Adams CE, Waldron S, Fuller RA, Macleod H (2004) Determining trophic niche width: a novel approach using stable isotope analysis. J Anim Ecol 73:1007–1012
Bearhop S, Phillips RA, McGill R, Cherel Y, Dawson DA, Croxall JP (2006) Stable isotopes indicate sex-specific and long-term individual foraging specialisation in diving seabirds. Mar Ecol Prog Ser 311:157–164. doi:10.3354/meps311157
Becker BH, Newman SH, Inglis S, Beissinger SR (2007) Diet-feather stable isotope (delta N-15 and delta C-13) fractionation in Common Murres and other seabirds. Condor 109:451–456. doi:10.1650/0010-5422%282007%29109%5B451:dsinac%5D2.0.co;2
Bond AL, Lavers JL (2011) Trace element concentrations in feathers of flesh-footed shearwaters (Puffinus carneipes) from across their breeding range. Arch Environ Contam Toxicol 61:318–326. doi:10.1007/s00244-010-9605-3
Bridge ES (2006) Influences of morphology and behavior on wing-molt strategies in seabirds. Mar Ornithol 34:7–19
Burger J, Gochfeld M (2000) Metal levels in feathers of 12 species of seabirds from Midway Atoll in the northern Pacific Ocean. Sci Total Environ 257:37–52. doi:10.1016/s0048-9697(00)00496-4
Carravieri A, Bustamante P, Churlaud C, Fromant A, Cherel Y (2014) Moulting patterns drive within-individual variations of stable isotopes and mercury in seabird body feathers: implications for monitoring of the marine environment. Mar Biol 161:963–968
Carrick R, Dunnet GM (1954) Breeding of the Fulmar Fulmarus glacialis. Ibis 96:356–370. doi:10.1111/j.1474-919X.1954.tb02329.x
Cherel Y, Hobson KA (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the Southern Ocean. Mar Ecol Prog Ser 329:281–287
Cherel Y, Hobson KA, Weimerskirch H (2000) Using stable-isotope analysis of feathers to distinguish moulting and breeding origins of seabirds. Oecologia 122:155–162. doi:10.1007/pl00008843
Cherel Y, Phillips RA, Hobson KA, McGill R (2006) Stable isotope evidence of diverse species-specific and individual wintering strategies in seabirds. Biol Lett 2:301–303. doi:10.1098/rsbl.2006.0445
Cherel Y, Quillfeldt P, Delord K, Weimerskirch H (2016) Combination of at-sea activity, geolocation and feather stable isotopes documents where and when seabirds moult. Front Ecol Evol. doi:10.3389/fevo.2016.00003
Cordes LS, Hedworth HE, Cabot D, Cassidy M, Thompson PM (2015) Parallel declines in survival of adult Northern Fulmars Fulmarus glacialis at colonies in Scotland and Ireland. Ibis 157:631–636. doi:10.1111/ibi.12255
Dauwe T, Bervoets L, Pinxten R, Blust R, Eens M (2003) Variation of heavy metals within and among feathers of birds of prey: effects of molt and external contamination. Environ Pollut 124:429–436. doi:10.1016/s0269-7491(03)00044-7
Dawson DA (2008) Genomic analysis of passerine birds using conserved microsatellite loci. Unpublished PhD Thesis. University of Sheffield, Department of Animal and Plant Sciences
Deben S, Angel Fernandez J, Aboal JR, Carballeira A (2012) Evaluation of different contour feather types for biomonitoring lead exposure in Northern goshawk (Accipiter gentilis) and tawny owl (Strix aluco). Ecotoxicol Environ Saf 85:115–119. doi:10.1016/j.ecoenv.2012.08.005
Dehnhard N, Voigt CC, Poisbleau M, Demongin L, Quillfeldt P (2011) Stable isotopes in southern rockhopper penguins: foraging areas and sexual differences in the non-breeding period. Polar Biol 34:1763–1773. doi:10.1007/s00300-011-1026-x
Dott HEM (1973) Fulmars at land in summer and autumn. Bird Study 20:221–225
Fisk AT, Tittlemier SA, Pranschke JL, Norstrom RJ (2002) Using anthropogenic contaminants and stable isotopes to assess the feeding ecology of Greenland sharks. Ecology 83:2162–2172. doi:10.2307/3072048
Font L, Nowell GM, Pearson DG, Ottley CJ, Willis SG (2007) Sr isotope analysis of bird feathers by TIMS: a tool to trace bird migration paths and breeding sites. J Anal At Spectrom 22:513–522. doi:10.1039/b616328a
Furness RW, Camphuysen CJ (1997) Seabirds as monitors of the marine environment. ICES J Mar Sci 54:726–737. doi:10.1006/jmsc.1997.0243
Furness RW, Muirhead SJ, Woodburn M (1986) Using bird feathers to measure mercury in the environment—relationships between mercury content and molt. Mar Pollut Bull 17:27–30. doi:10.1016/0025-326x(86)90801-5
Furness RW et al (2006) Techniques to link individual migration patterns of seabirds with diet specialization, condition and breeding performance. Ardea 94:631–638
Gargallo G (2013) Feather selection and replacement patterns demonstrate that Goldfinches Carduelis carduelis fix postjuvenile moult extent prior to moult initiation. J Ornithol 154:219–230. doi:10.1007/s10336-012-0888-1
Ginn HB, Melville DS (1983) Moult in birds. British Trust for Ornithology, Tring, Hertfordshire
Goede AA, De Bruin M (1984) The use of feather parts as a monitor for metal pollution. Environ Pollut Ser B Chem Phys 8:281–298. doi:10.1016/0143-148x(84)90028-4
Gómez-Díaz E, González-Solís J (2007) Geographic assignment of seabirds to their origin: combining morphologic, genetic, and biogeochemical analyses. Ecol Appl 17:1484–1498
Griffiths R, Double MC, Orr K, Dawson RJ (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Harris MP, Daunt F, Newell M, Phillips RA, Wanless S (2010) Wintering areas of adult Atlantic puffins Fratercula arctica from a North Sea colony as revealed by geolocation technology. Mar Biol 157:827–836. doi:10.1007/s00227-009-1365-0
Hedd A, Montevecchi WA (2006) Diet and trophic position of Leach’s storm-petrel Oceanodroma leucorhoa during breeding and moult, inferred from stable isotope analysis of feathers. Mar Ecol Prog Ser 322:291–301. doi:10.3354/meps322291
Hedenström A, Sunada S (1999) On the aerodynamics of moult gaps in birds. J Exp Biol 202:67–76
Hemborg C, Sanz JJ, Lundberg A (2001) Effects of latitude on the trade-off between reproduction and moult: a long-term study with pied flycatcher. Oecologia 129:206–212. doi:10.1007/s004420100710
Hinsley SA, Rothery P, Ferns PN, Bellamy PE, Dawson A (2003) Wood size and timing of moult in birds: potential consequences for plumage quality and bird survival. Ibis 145:337–340. doi:10.1046/j.1474-919X.2003.00167.x
Hobson KA (1999) Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia 120:314–326. doi:10.1007/s004420050865
Hobson KA, Bond AL (2012) Extending an indicator: year-round information on seabird trophic ecology from multiple-tissue stable-isotope analyses. Mar Ecol Prog Ser 461:233–243. doi:10.3354/meps09835
Hobson KA, Norris DR (2008) Animal migration: a context for using new techniques and approaches. In: Hobson KA, Wassenaar LI (eds) Tracking animal migration with stable isotopes, vol 2. Academic Press, London. doi:10.1016/s1936-7961(07)00001-2
Hooker SK, Iverson SJ, Ostrom P, Smith SC (2001) Diet of northern bottlenose whales inferred from fatty-acid and stable-isotope analyses of biopsy samples. Can J Zool 79:1442–1454. doi:10.1139/cjz-79-8-1442
Inger R, Bearhop S (2008) Applications of stable isotope analyses to avian ecology. Ibis 150:447–461. doi:10.1111/j.1474-919X.2008.00839.x
Jaeger A, Blanchard P, Richard P, Cherel Y (2009) Using carbon and nitrogen isotopic values of body feathers to infer inter- and intra-individual variations of seabird feeding ecology during moult. Mar Biol 156:1233–1240. doi:10.1007/s00227-009-1165-6
Jaeger A, Lecomte VJ, Weimerskirch H, Richard P, Cherel Y (2010) Seabird satellite tracking validates the use of latitudinal isoscapes to depict predators’ foraging areas in the Southern Ocean. Rapid Commun Mass Spectrom 24:3456–3460. doi:10.1002/rcm.4792
Jaspers VLB, Covaci A, Deleu P, Neels H, Eens M (2008) Preen oil as the main source of external contamination with organic pollutants onto feathers of the common magpie (Pica pica). Environ Int 34:741–748. doi:10.1016/j.envint.2007.12.002
Jerez S, Motas M, Jose Palacios M, Valera F, Javier Cuervo J, Barbosa A (2011) Concentration of trace elements in feathers of three Antarctic penguins: geographical and interspecific differences. Environ Pollut 159:2412–2419. doi:10.1016/j.envpol.2011.06.036
Kopp M, Peter H-U, Mustafa O, Lisovski S, Ritz MS, Phillips RA, Hahn S (2011) South polar skuas from a single breeding population overwinter in different oceans though show similar migration patterns. Mar Ecol Prog Ser 435:263–267. doi:10.3354/meps09229
Kouwenberg A-L, Hipfner JM, McKay DW, Storey AE (2013) Corticosterone and stable isotopes in feathers predict egg size in Atlantic Puffins Fratercula arctica. Ibis 155:413–418. doi:10.1111/ibi.12030
Kubetzki U, Garthe S, Fifield D, Mendel B, Furness RW (2009) Individual migratory schedules and wintering areas of northern gannets. Mar Ecol Prog Ser 391:257–265. doi:10.3354/meps08254
Langin KM, Reudink MW, Marra PP, Norris DR, Kyser TK, Ratcliffe LM (2007) Hydrogen isotopic variation in migratory bird tissues of known origin: implications for geographic assignment. Oecologia 152:449–457. doi:10.1007/s00442-007-0669-3
Larson KW, Hobson KA (2009) Assignment to breeding and wintering grounds using stable isotopes: a comment on lessons learned by Rocque. J Ornithol 150:709–712. doi:10.1007/s10336-009-0408-0
Leat EHK et al (2013) Influence of wintering area on persistent organic pollutants in a breeding migratory seabird. Mar Ecol Prog Ser 491:277. doi:10.3354/meps10455
Lewis S, Elston DA, Daunt F, Cheney B, Thompson PM (2009) Effects of extrinsic and intrinsic factors on breeding success in a long lived seabird. Oikos 118:521–528
Mackley EK, Phillips RA, Silk JR, Wakefield ED, Afanasyev V, Furness RW (2011) At-sea activity patterns of breeding and nonbreeding white-chinned petrels Procellaria aequinoctialis from South Georgia. Mar Biol 158:429–438
Moreno R, Jover L, Diez C, Sanpera C (2011) Seabird feathers as monitors of the levels and persistence of heavy metal pollution after the Prestige oil spill. Environ Pollut 159:2454–2460. doi:10.1016/j.envpol.2011.06.033
Pearson SF, Levey DJ, Greenberg CH, del Rio CM (2003) Effects of elemental composition on the incorporation of dietary nitrogen and carbon isotopic signatures in an omnivorous songbird. Oecologia 135:516–523. doi:10.1007/s00442-003-1221-8
Phillips RA, Silk JRD, Croxall JP, Afanasyev V, Briggs DR (2004) Accuracy of geolocation estimates for flying seabirds. Mar Ecol Prog Ser 266:265–272. doi:10.3354/meps266265
Phillips RA, Bearhop S, McGill RAR, Dawson DA (2009) Stable isotopes reveal individual variation in migration strategies and habitat preferences in a suite of seabirds during the nonbreeding period. Oecologia 160:795–806. doi:10.1007/s00442-009-1342-9
Polizzi PS, Chiodi Boudet LN, Romero MB, Denuncio PE, Rodriguez DH, Gerpe MS (2013) Fine scale distribution constrains cadmium accumulation rates in two geographical groups of Franciscana dolphin from Argentina. Mar Pollut Bull 72:41–46. doi:10.1016/j.marpolbul.2013.05.003
Quillfeldt P, McGill RAR, Furness RW (2005) Diet and foraging areas of Southern Ocean seabirds and their prey inferred from stable isotopes: review and case study of Wilson’s storm-petrel. Mar Ecol Prog Ser 295:295–304. doi:10.3354/meps295295
Quinn LR (2014) Intra- and inter-colony differences in non-breeding strategies in the Northern Fulmar, Fulmarus glacialis. Unpublished PhD Thesis, University of Aberdeen
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org
Ramos R, González-Solís J (2012) Trace me if you can: the use of intrinsic biogeochemical markers in marine top predators. Front Ecol Environ 10:258–266. doi:10.1890/110140
Ramos R, Militao T, Gonzalez-Solis J, Ruiz X (2009) Moulting strategies of a long-distance migratory seabird, the Mediterranean Cory’s Shearwater Calonectris diomedea diomedea. Ibis 151:151–159. doi:10.1111/j.1474-919X.2008.00877.x
Ryder TB, Wolfe JD (2009) The current state of knowledge on molt and plumage sequences in selected neotropical bird families: a review. Ornitologia Neotropical 20:1–18
Seminoff JA, Benson SR, Arthur KE, Eguchi T, Dutton PH, Tapilatu RF, Popp BN (2012) Stable isotope tracking of endangered sea turtles: validation with satellite telemetry and delta N-15 analysis of amino acids. PLoS One. doi:10.1371/journal.pone.0037403
Thompson DR, Furness RW (1995) Stable-isotope ratios of carbon and nitrogen in feathers indicate seasonal dietary shifts in northern fulmars. Auk 112(2):493–498
Thompson P, Ollason J (2001) Lagged effects of ocean climate change on fulmar population dynamics. Nature 413:417–420
Thompson DR, Furness RW, Lewis SA (1995) Diets and long term changes in delta N15 and delta C13 values in Northern Fulmars Fulmarus glacialis from two NE Atlantic colonies. Mar Ecol Prog Ser 125:3–11. doi:10.3354/meps125003
van Franeker JA (2004) Mass mortality of fulmars in the southern North Sea. Nieuwsbrief NZG 5:6–7
van Franeker JA et al (2011) Monitoring plastic ingestion by the northern fulmar Fulmarus glacialis in the North Sea. Environ Pollut 159:2609–2615. doi:10.1016/j.envpol.2011.06.008
Venables WN, Ripley BD (2002) Modern applied statistics with S. Springer, New York
Weimerskirch H (1991) Sex-specific differences in molt strategy in relation to breeding in the wandering albatross. Condor 93:731–737. doi:10.2307/1368205
Acknowledgments
We thank Claire Deacon, Gareth Norton and Andrea Raab for help with laboratory work at the University of Aberdeen, and Barry Thornton and Gillian Martin for running stable isotope analysis at the James Hutton Institute. Thanks to all involved in the collection and processing of dead fulmars through the North Sea plastic pollution project at IMARES, with special thanks to Jens-Kjeld Jensen, Bergur Olsen and Elisa Bravo Rebolledo for samples from the Faroe Islands and Susanne Kühn for those from Iceland. Thanks to Orkney Islands Council for access to Eynhallow and to all the fieldworkers involved in deployment and recovery of the GLS tags. All ringing work was carried out under permit from the BTO, and feather sampling was carried out under licence from the Home Office. We are grateful to James Fox of Migrate Technologies for recovering data from GLS loggers which would not download, and Richard Phillips and Janet Silk of BAS for advice on GLS analysis. We thank Deborah Dawson of the NERC Biomolecular Analysis Facility, University of Sheffield and Stuart Piertney of University of Aberdeen for molecular sexing of the fulmars. Lucy Quinn was supported by a NERC Studentship and additional funding to support fieldwork was gratefully received from Talisman Energy (UK) Ltd. We thank Yves Cherel and two anonymous reviewers for their constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Y. Cherel.
Reviewed by Undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Quinn, L.R., Meharg, A.A., van Franeker, J.A. et al. Validating the use of intrinsic markers in body feathers to identify inter-individual differences in non-breeding areas of northern fulmars. Mar Biol 163, 64 (2016). https://doi.org/10.1007/s00227-016-2822-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-016-2822-1