Abstract
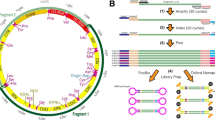
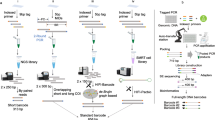
Based on only a handful of mitochondrial and nuclear loci, the phylogenetic relationships within the genus Acropora have been unclear. However, with the new sequencing technology of massively parallel sequencing (MPS), the inter-specific relationships within Acropora may be resolved. We performed multiplex sequencing of the mitochondrial genome of eleven Acropora species representing different groups using the Illumina Solexa platform. Mitochondrial genomes were sequenced from long PCR-amplified templates ligated with different index sequences (~9 kbp) and analyzed using the mitochondrial genome of Acropora tenuis as a reference. A total of 75 million read outputs in one Illumina lane were obtained, with mapping results having coverage up to 44,000-fold. Assembly results of multiplex samples confirmed with Sanger sequencing produced <0.03 % error. Aligning the eleven mitochondrial genomes with the reference sequence revealed only 110 phylogenetically informative sites over the mitochondrial genome. The largest pairwise genetic distance observed was in the putative control region (0.022). A comparison of two phylogenetic trees based on the whole mitochondrial genome and control region showed that the former tree produces a higher resolution of phylogenetic relationships. In this study, we demonstrated the first case of sequencing cnidarian mitochondrial genomes by using multiplex MPS and applying it in a phylogenomic analyses.


Similar content being viewed by others
References
Ansorge W, Voss H, Zimmermann J (1997) DNA sequencing strategies. Wiley, New York
Babcock R (1995) Synchronous multispecific spawning on coral reefs: potential for hybridization and roles of gamete recognition. Reprod Fert Develop 7:943–950
Babcock RC, Bull GD, Harrison PL, Heyward AJ, Oliver JK, Wallace CC, Willis BL (1986) Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar Biol 90:379–394
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics: btu170
Chen CA, Wallace CC, Wolstenholme J (2002) Analysis of the mitochondrial 12S rRNA gene supports a two-clade hypothesis of the evolutionary history of scleractinian corals. Mol Phylogenet Evol 23:137–149
Chen IP, Tang CY, Chiou CY, Hsu JH, Wei NV, Wallace CC, Muir P, Wu H, Chen CA (2009) Comparative analyzes of coding and noncoding DNA regions indicate that Acropora (Anthozoa: Scleractina) possesses a similar evolutionary tempo of nuclear vs. mitochondrial genomes as in plants. Mar Biotechnol 11:141–152
Cronn R, Liston A, Parks M, Gernandt DS, Shen R, Mockler T (2008) Multiplex sequencing of plant chloroplast genomes using Solexa sequencing-by-synthesis technology. Nucleic Acids Res 36:e122
Deng W, Maust BS, Nickle DC, Learn GH, Liu Y, Heath L, Sergei L, Pond K, Mullins JI (2010) DIVEIN: a web server to analyze phylogenies, sequence divergence, diversity, and informative sites. Biotechniques 48:405–408
Doorduin L, Gravendee B, Lammers Y, Ariyurek Y, Woeng TCA, Vrieling K (2011) The complete chloroplast genome of 17 individuals of pest species Jacobaea vulgaris: SNPs, microsatellites and barcoding markers for population and phylogenetic studies. DNA Res 18:93–105
Ekblom R, Galindo J (2011) Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity 107:1–15
Faircloth BC, Sorenson L, Santini F, Alfaro ME (2013) A phylogenomic perspective on the radiation of ray-finned fishes based upon targeted sequencing of ultraconserved elements (UCEs). PLoS One 8:e65923
Feldmeyer B, Hoffmeier K, Pfenninger M (2010) The complete mitochondrial genome of Radix balthica (Pulmonata, Basommatophora), obtained by low coverage shot gun next generation sequencing. Mol Phylogenet Evol 57:1329–1333
Figueroa DF, Baco AR (2014) Complete mitochondrial genomes elucidate phylogenetic relationships of the deep-sea octocoral families Coralliidae and Paragorgiidae. Deep-Sea Res Part II 99:83–91
Fukami H, Omori M, Hatta M (2000) Phylogenetic relationships in the coral family Acroporidae, reassessed by inference from mitochondrial genes. Zool Sci 17:689–696
Fukami H, Budd AF, Levitan DR, Jara J, Kersanach R, Knowlton N (2004) Geographic differences in species boundaries among members of the Montastraea Annularis complex based on molecular and morphological markers. Evolution 58:324–337
Fukami H, Chen CA, Budd AF, Collins A, Wallace CC, Chuang YY, Chen C, Dai CF, Iwao K, Sheppard C, Knowlton N (2008) Mitochondrial and nuclear genes suggest that stony corals are monophyletic but most families of stony corals are not (Order Scleractinia, Class Anthozoa, Phylum Cnidaria). PLoS One 3:e3222
Harrison PL, Babcock RC, Bull GD, Oliver JK, Wallace CC, Willis BL (1984) Mass spawning in tropical reef corals. Science 223:1186–1189
Hatta M, Fukami H, Wang W, Omori M, Shimoike K, Hayashibara T, Ina Y, Sugiyama T (1999) Reproductive and genetic evidence for a reticulate evolutionary history of mass-spawning corals. Mol Biol Evol 16:1607–1613
Hellberg ME (2006) No variation and low synonymous substitution rates in coral mtDNA despite high nuclear variation. BMC Evol Biol 6:24
Huang D, Meier R, Todd PA, Chou LM (2008) Slow mitochondrial COI sequence evolution at the base of the metazoan tree and its implications for DNA barcoding. J Mol Evol 66:167–174
Huang D, Licuanan WY, Baird AH, Fukami H (2011) Cleaning up the “Bigmessidae”: molecular phylogeny of scleractinian corals from Faviidae, Merulinidae, Pectiniidae and Trachyphylliidae. BMC Evol Biol 11:37
Hudson ME (2008) Sequencing breakthroughs for genomic ecology and evolutionary biology. Mol Ecol Resour 8:3–17
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogeny. Bioinformatics 17:754–755
Jex AR, Hu M, Littlewood DTJ, Waeschenbach A, Gasser RB (2008) Using 454 technology for long-PCR based sequencing of the complete mitochondrial genome from single Haemonchus contortus (Nematoda). BMC Genomics 9:11
Jex AR, Waeschenbach A, Hu M, van Wyk JA, Beveridge I, Littlewood DTJ, Gasser RB (2009a) The mitochondrial genomes of Ancylostoma caninum and Bunostomum phlebotomum—two hookworms of animal health and zoonotic importance. BMC Genomics 10:79
Jex AR, Hall RS, Littlewood DTJ, Gasser RB (2009b) An integrated pipeline for next-generation sequencing and annotation of mitochondrial genomes. Nucleic Acids Res 382:522–533
Kitahara MV, Cairns SD, Stolarski J, Blair D, Miller DJ (2010) A comprehensive phylogenetic analysis of the Scleractinia (Cnidaria, Anthozoa) based on mitochondrial CO1 sequence data. PLoS One 5:e11490
Kitahara MV et al (2014) The “naked coral” hypothesis revisited—evidence for and against scleractinian monophyly. PLoS One 9:e94774
Lin MF, Luzon KS, Licuanan WY, Ablan-Lagman MC, Chen CA (2011) Seventy-four universal primers for characterizing the complete mitochondrial genomes of scleractinian corals (Cnidaria; Anthozoa). Zool Stud 50:513–524
Lin MF, Kitahara MV, Luo H, Tracey D, Geller J, Fukami H, Miller DJ, Chen CA (2014) Mitochondrial genome rearrangements in the scleractinia/corallimorpharia complex: implications for coral phylogeny. Genome Biol Evol 6:1086–1095
McFadden CS, Benayahu E, Thoma JN, Nevarez PA, France SC (2011) Limitations of mitochondrial gene barcoding in Octocorallia. Mol Ecol Resour 11:19–31
Miller JR, Koren S, Sutton G (2010) Assembly algorithms for next-generation sequencing data. Genomics 95:315–327
Nakajima Y, Nishikawa A, Iguchi A, Sakai K (2012) The population genetic approach delineates the species boundary of reproductively isolated corymbose acroporid corals. Mol Phylogent Evol 63:527–531
Odorico DM, Miller DJ (1997) Variation in the ribosomal internal transcribed spacers and 5.8 s rDNA among five species of Acropora (Cnidaria; Scleractinia): patterns of variation consistent with reticulate evolution. Mol Biol Evol 14:465–473
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Richards Z, Miller DJ, Wallaces CC (2013) Molecular phylogenetics of geographically restricted Acropora species: implications for threatened species conservation. Mol Phylogenet Evol 69:837–851
Romano SL, Palumbi SR (1996) Evolution of scleractinian corals inferred from molecular systematics. Science 271:640–642
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Shearer TL, van Oppen MJH, Romano SL, Wörheide G (2002) Slow mitochondrial DNA sequence evolution in the Anthozoa (Cnidaria). Mol Ecol 11:2475–2487
Shendure J, Ji H (2008) Next-generation DNA sequencing. Nature biotechnol 26:1135–1145
Smith AM, Heisler LE, St Onge RP, Farias-Hesson E, Wallace IM, Bodeau J, Harris AN, Perry KM, Giaever G, Pourmand N, Nislow C (2010) Highly-multiplexed barcode sequencing: an efficient method for parallel analysis of pooled samples. Nucleic Acids Res 38:e142
Takahiro K (2003) Bias and artifacts in multitemplate polymerase chain reactions (PCR). J Biosci Bioeng 96:317–323
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Timmermans M, Dodsworth S, Culverwell CL, Bocak L, Ahrens D, Littlewood DT, Pons J, Vogler AP (2010) Why barcode? High-throughput multiplex sequencing of mitochondrial genomes for molecular systematics. Nucleic Acids Res 38:e197
Tseng CC, Wallace CC, Chen CA (2005) Mitogenomic analysis of Montipora cactus and Anacropora matthai (Cnidaria; Scleractinia; Acroporidae) indicates an unequal rate of mitochondrial evolution among Acroporidae corals. Coral Reefs 24:502–508
Tu J, Ge Q, Wang S, Wang L, Sun B, Yang Q, Bai Y, Lu Z (2012) Pair-barcode high-throughput sequencing for large-scale multiplexed sample analysis. BMC Genomics 13:43
van Oppen MJH, Willis BL, Miller DJ (1999) Atypically low rate of cytochrome b evolution in the scleractinian coral genus Acropora. Proc R Soc Lond B 266:179–183
van Oppen MJH, Catmull J, McDonald BJ, Hislop NR, Hagerman PJ, Miller DJ (2000a) The mitochondrial genome of Acropora tenuis (Cnidaria; Scleractinia) contains a large group I intron and a candidate control region. J Mol Evol 55:1–13
van Oppen MJH, Willis BL, Vugt HWJAV, Miller DJ (2000b) Examination of species boundaries in the Acropora cervicornis group (Scleractinia, Cnidaria) using nuclear DNA sequence analyzes. Mol Ecol 9:1363–1373
van Oppen MJH, McDonald BJ, Willis BL, Miller DJ (2001) The evolutionary history of the coral genus Acropora (Scleractinia, Cnidaria) based on a mitochondrial and a nuclear marker: reticulation, incomplete lineage sorting, or morphological convergence? Mol Biol Evol 18:1315–1329
van Oppen MJH, Willis BL, Van RT, Miller DJ (2002) Spawning times, reproductive compatibilities and genetic structuring in the Acropora aspera group: evidence for natural hybridization and semi-permeable species boundaries in corals. Mol Ecol 11:1363–1376
Veron JEN (2000) Corals of the world. Australian Institute of Marine Science, Townsville
Veron JEN, Wallace CC (1984). Scleractinia of eastern Australia, Part V. Family Acroporidae AIMS Monogr Ser 6: 485
Vollmer SV, Palumbi SR (2002) Hybridization and the evolution of coral reef diversity. Science 296:2023–2025
Wallace CC (1999) Staghorn corals of the world: a revision of the genus Acropora. CSIRO Publishing, Collingwood
Wallace CC, Willis BL (1994) Systematics of the coral genus Acropora: implications of new biological findings for species concepts. Annu Rev Ecol Syst 25:237–262
Wallace CC, Wolstenholme J (1998) Revision of the coral genus Acropora (Scleractinia: Astrocoeniina: Acroporidae) in Indonesia. Zool J Linn Soc 123:199–384
Wang M, Sun J, Li J, Qiu JW (2013) Complete mitochondrial genome of the brain coral Platygyra carnosus. Mitochondrial DNA 24:194–195
Williams ST, Foster PG, Littlewood DTJ (2014) The complete mitochondrial genome of a turbinid vetigastropod from MiSeq Illumina sequencing of genomic DNA and steps towards a resolved gastropod phylogeny. Gene 533:38–47
Willis BL, Babcock RC, Harrison PL, Wallace CC (1997) Experimental hybridization and breeding incompatibilities within the mating systems of mass spawning reef corals. Coral Reefs 16:S53–S65
Willis BL, van Oppen MJH, Miller DJ, Vollmer SV, Ayre DJ (2006) The role of hybridization in the evolution of reef corals. Ann Rev Ecol Evol Syst 37:489–517
Wolstenholme JK (2004) Temporal reproductive isolation and gametic compatibility are evolutionary mechanisms in the Acropora humilis species group (Cnidaria; Scleractinia). Mar Biol 144:567–582
Wolstenholme JK, Wallace CC, Chen CA (2003) Species boundaries within the Acropora humilis species group (Cnidaria; Scleractinia): a morphological and molecular interpretation of evolution. Coral Reefs 22:155–166
Acknowledgments
This project was funded by the National Science Council (2010–2012), Academia Sinica Thematic Grant (2006–2010), and the Biodiversity Research Centre, Academia Sinica (BRCAS) to CAC. We would like to thank the staffs of Penghu Marine Biological Research Centre (PMBRC)-BRCAS joint Marine Laboratory, BRCAS Green Island Reef Laboratory (GIRL) Green Island Marine Station, and Kenting National Park for assistance with fieldwork. Special thanks go to CREEG members and the David Miller Group for intellectual inputs to this paper, and to Tyler McCraney and Buford Pruitt, Jr. for English language editing. We also greatly thank three anonymous reviewers and the communicating editor for useful comments and suggestions to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Riginos.
Shang-Yin Vanson Liu and Chia-Ling Carynn Chan are co-first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, SY.V., Chan, CL.C., Hsieh, H.J. et al. Massively parallel sequencing (MPS) assays for sequencing mitochondrial genomes: the phylogenomic implications for Acropora staghorn corals (Scleractinia; Acroporidae). Mar Biol 162, 1383–1392 (2015). https://doi.org/10.1007/s00227-015-2657-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-015-2657-1