Abstract
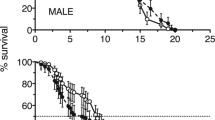
Lifetime spermatophore (sperm packet) production, which can provide valuable information about male energetics and mating, is not known for any species of copepod. Spermatophore production could limit reproduction in species in which females require multiple matings to remain fertilized and in populations that often have adult sex ratios highly skewed toward females. Spermatophore production rates were measured in the laboratory for two species of calanoid copepods, Acartia tonsa and Acartia hudsonica, from time of maturation until death, and under three food regimes (high, low, and no food). Rates of spermatophore production in A. tonsa were independent of food treatment. By contrast, in A. hudsonica, spermatophore production was significantly greater under high food than in low or no food. Spermatophore production decreased significantly with age in both species and the majority of males ceased producing spermatophores halfway through their adult lives. This pattern occurred regardless of food treatment, except for in A. hudsonica under low-food conditions: low production rates in younger males were compensated by continued reproduction into old age. Therefore, there was no difference in the total lifetime number of spermatophores produced by males of A. hudsonica fed at high- or low-food conditions. In the absence of food, however, significantly fewer spermatophores were produced. In A. tonsa, there was no difference among food treatments in the total number of spermatophores produced over a male’s life. The effect of these low rates of spermatophore production on fertilization was evident in the field. During 2 years of weekly collections, at no point in time were all females in the population fertilized. We conclude that low rates of spermatophore production over a lifetime and the short reproductive period of males contribute to the low frequencies of mated females in field populations.






Similar content being viewed by others
References
Ali AK, Primicerio R, Folstad I, Liljedal S, Berge J (2009) Morphological correlates of mating frequency and clutch size in wild caught female Eudiaptomus graciloides (Copepoda: Calanoida). J Plankton Res 31:389–397
Anstensrud M (1990) Mating strategies of two parasitic copepods Lernaeocera branchialis (L.) (Pennellidae) and Lepeophtheirus pectoralis (Müller) (Caligidae) on flounder: polygamy, sex-specific age at maturity and sex ratio. J Exp Mar Biol Ecol 136:141–158
Anstensrud M (1992) Mate guarding and mate choice in two copepods, Lernaeocera branchialis (L.)(Pennellidae) and Lepeophtheirus pectoralis (Müller) (Caligidae), parasitic on flounder. J Crustac Biol 12:31–40
Arnqvist G, Nilsson T (2000) The evolution of polyandry: multiple mating and female fitness in insects. Anim Behav 60:145–164
Bagøien E, Kiørboe T (2005) Blind dating-mate finding in planktonic copepods. III. Hydromechanical communication in Acartia tonsa. Mar Ecol Prog Ser 300:129–133
Bateman AJ (1948) Intrasexual selection in Drosophila. Heredity 2:349–368
Bergreen U, Hansen B, Kiørboe T (1988) Food size spectra, ingestion and growth of the copepod Acartia tonsa during development: implication for determination of copepod production. Mar Biol 99:341–352
Besiktepe S, Dam HG (2002) Coupling of ingestion and defecation as a function of diet in the calanoid copepod Acartia tonsa. Mar Ecol Prog Ser 229:151–164
Birkhead TR, Møller AP (1998) Sperm competition and sexual selection. Academic Press, San Diego
Boggs CL (1990) A general model of the role of male-donated nutrients in female insects’ reproduction. Am Nat 136:598–617
Boggs CL (1995) Male nuptial gifts: phenotypic consequences and evolutionary implications. In: Leather SR, Hardie J (eds) Insect reproduction. CRC Press, Boca Raton, pp 215–242
Bonduriansky R, Brassil CE (2005) Reproductive ageing and sexual selection on male body size in a wild population of antler flies (Protopiophila litigara). J Evol Biol 18:1332–1340
Bradbury JW, Andersson MB (1987) Sexual selection: testing the alternatives. Wiley, New York
Burris ZP, Dam HG (2014) Female mating status affects mating and male mate-choice in the copepod genus Acartia. J Plankton Res. doi:10.1093/plankt/fbu090
Burton RS (1985) Mating system of the intertidal copepod Tigriopus californicus. Mar Biol 86:247–252
Cardoso MZ, Gilbert LE (2007) A male gift to its partner? Cyanogenic glycosides in the spermatophore of longwing butterflies (Heliconius). Naturwissenschaften 94:39–42
Ceballos S, Kiørboe T (2010) First evidences of sexual selection by mate choice in marine zooplankton. Oecologia 164:627–635
Ceballos S, Kiørboe T (2011) Senescence and sexual selection in a pelagic copepod. PLoS One. doi:10.1371/journal.pone.0018870
Ceballos S, Sichlau MH, Heuschele J, Kiørboe T (2014) Low fertilization rates in a pelagic copepod caused by sexual selection? J Plankton Res 36:736–742
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton
Choi KH, Kimmerer WJ (2009) Mating success and its consequences for population growth in an estuarine copepod. Mar Ecol Prog Ser 377:183–191
Clutton-Brock TH (1988) Reproductive success. University of Chicago Press, Chicago
Conover RJ (1956) Oceanography of Long Island Sound, 1952–1954. VI. Biology of Acartia clausi and A. tonsa. Bull Bingham Oceanogr Coll 15:6–233
Dam HG, Peterson WT, Bellantoni DC (1994) Seasonal feeding and fecundity of the calanoid copepod Acartia tonsa in Long Island Sound: is omnivory important to egg production? Hydrobiologia 292:191–199
Deevey GB (1960) The zooplankton of the surface waters of the Delaware Bay region. Bull Bingham Oceanogr Coll 17:5–53
Defaye D, Cuoc C, Brunet M (2000) Genital structures and spermatophore placement in female Paradiaptominae (Copepoda, Calanoida, Diaptomidae). J Crustac Biol 20:245–261
Dewsbury DA (1982) Ejaculate cost and male choice. Am Nat 119:601–610
Durbin EG, Durbin AG, Campbell RG (1992) Body size and egg production in the marine copepod Acartia hudsonica during a winter–spring diatom bloom in Narragansett Bay. Limnol Oceanogr 37:342–360
Ferrari F, Dojiri M (1987) The calanoid copepod Euchaeta antarctica from the Southern Ocean Atlantic sector midwater trawls, with observations on spermatophore dimorphism. J Crustac Biol 7:458–480
Finiguerra MB, Dam HG, Avery DE, Burris Z (2013) Sex-specific tolerance to starvation in the copepod Acartia tonsa. J Exp Mar Biol Ecol 446:17–21
Guillard RR (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum Press, New York, pp 29–60
Gulati R, Demott W (1997) The role of food quality for zooplankton: remarks on the state-of-the-art, perspectives and priorities. Freshw Biol 38:753–768
Gwynne DT (1997) The evolution of edible sperm sacs and other forms courtship feeding in crickets, katydids, and their kin (Orthoptera: Ensifera). In: Choe JC, Crespi BJ (eds) The evolution of mating systems in insects and arachnids. Cambridge University Press, Cambridge, pp 110–129
Gwynne DT (2008) Sexual conflict over nuptial gifts in insects. Annu Rev Entomol 53:83–101
Hammer RM (1978) Scanning electron microscope study of the spermatophore of Acartia tonsa (Copepoda: Calanoida). Trans Am Microsc Soc 97:386–389
Heuch PA, Schram TA (1996) Male mate choice in a natural population of the parasitic copepod Lernaeocera branchialis (Copepoda: Pennellidae). Behaviour 133:221–239
Heuschele J, Kiørboe T (2012) The smell of virgins: mating status of females affects male swimming behaviour in Oithona davisae. J Plankton Res 34:929–935
Heuschele J, Ceballos S, Anderson Borg CM, Bjærke O, Isari S, Lasley-Rasher R, Lindehoff E, Souissi A, Souissi S, Titelman J (2014) Non-consumptive effects of predator presence on copepod reproduction: insights from a mesocosm experiment. Mar Biol. doi:10.1007/s00227-014-2449-z
Hirschfeld HS (1974) Some observations on the breeding of Pseudocalanus in the laboratory and comparisons with other calanoid species. B.Sc. Hons. thesis, Dalhousie University, Halifax, Nova Scotia
Hirst AG, Kiørboe T (2002) Mortality of marine planktonic copepods: global rates and patterns. Mar Ecol Prog Ser 230:195–209
Hopkins CCE (1978) The male genital system, and spermatophore production and function in Euchaeta norvegica Boeck (Copepoda: Calanoida). J Exp Mar Biol Ecol 35:197–231
Hopkins CCE (1982) The breeding biology of Euchaeta norvegica (Boeck) (Copepoda: Calanoida) in Loch Etive, Scotland: assessment of breeding intensity in terms of seasonal cycles in the sex ratio, spermatophore attachment, and egg-sac production. J Exp Mar Biol Ecol 60:91–102
Hopkins CCE, Machin D (1977) Patterns of spermatophore distribution and placement in Euchaeta norvegica (Copepoda: Calanoida). J Mar Biol Assoc UK 57:113–131
Ianora A, Poulet SA (1993) Egg viability in the copepod Temora stylifera. Limnol Oceanogr 38:1615–1626
Ianora A, Poulet SA, Miralto A (1995) A comparative study of the inhibitory effect of diatoms on the reproductive biology of the copepod Temora stylifera. Mar Biol 121:533–539
Ianora A, Poulet SA, Miralto A, Grottoli R (1996) The diatom Thalassiosira rotula affects reproductive success in the copepod Acartia clausi. Mar Biol 125:279–286
Ianora A, Miralto A, Buttino I, Romano G, Poulet SA (1999) First evidence of some dinoflagellates reducing male copepod fertilization capacity. Limnol Oceanogr 44:147–153
Irigoien X, Harris RP, Verheye HM, Joly P, Runge J, Starr M, Davidson R (2002) Copepod hatching success in marine ecosystems with high diatom concentrations. Nature 419:387–389
Kiørboe T (2006) Sex, sex-ratios, and the dynamics of pelagic copepod populations. Oecologia 148:40–50
Kiørboe T (2007) Mate finding, mating, and population dynamics in a planktonic copepod Oithona davisae: there are too few males. Limnol Oceanogr 52:1511–1522
Kiørboe T, Bagøien E (2005) Motility patterns and mate encounter rates in planktonic copepods. Limnol Oceanogr 50:1999–2007
Lazzaretto I, Salvato B, Libertini A (1990) Evidence of chemical signaling in Tigriopus fulvus (Copepoda, Harpacticoida). Crustaceana 59:171–179
Lee WY, McAlice BJ (1979) Seasonal succession and breeding cycles of three species of Acartia (Copepoda: Calanoida) in a Maine estuary. Estuaries 2:228–235
Lee ET, Wang J (2003) Statistical methods for survival data analysis. Wiley, New York
Lee RF, Hagen W, Kattner G (2006) Lipid storage in marine zooplankton. Mar Ecol Prog Ser 307:273–306
Maps F, Runge JA, Zakardjian B, Joly P (2005) Egg production and hatching success of Temora longicornis (Copepoda, Calanoida) in the southern Gulf of St. Lawrence. Mar Ecol Prog Ser 285:117–128
Marshall SM, Orr AP (1952) On the biology of Calanus finmarchicus. VII. Factors affecting egg production. J Mar Biol Assoc UK 30:527–549
Mauchline J (1998) The biology of calanoid copepods. In: Blaxter J, Southward A (eds) Advances in marine biology. Academic Press, San Diego, p 710
McKean KA, Nunney L (2001) Increased sexual activity reduces male immune function in Drosophila melanogaster. Proc Natl Acad Sci USA 98:7904–7909
Miralto A, Ianora A, Poulet SA (1995) Food type induces different reproductive responses in the copepod Centropages typicus. J Plankton Res 17:1521–1534
Müller-Navarra D, Lampert W (1996) Seasonal patterns of food limitation in Daphnia galeata: separating food quantity and food quality effects. J Plankton Res 18:1137–1157
Ohtsuka S, Huys R (2001) Sexual dimorphism in calanoid copepods: morphology and function. Hydrobiologia 453:441–466
Parrish KK, Wilson DF (1978) Fecundity studies on Acartia tonsa (Copepoda: Calanoida) in standardized culture. Mar Biol 46:65–81
Peterson WT (1986) The effects of seasonal variations in stratification on plankton dynamics in Long Island Sound. In: Bowman MJ (ed) Tidal mixing and plankton dynamics. Springer, New York, pp 297–320
Peterson WT (2001) Patterns in stage duration and development among marine and freshwater calanoid and cyclopoid copepods: a review of rules, physiological constraints, and evolutionary significance. Hydrobiologia 454:91–105
Rodríguez-Graña L, Calliari D, Tiselius P, Hansen BW, Sköld HN (2010) Gender specific ageing and non-Mendelian inheritance of oxidative damage in marine copepods. Mar Ecol Prog Ser 401:1–13
Saether BE (1990) Age-specific variation in reproductive performance of birds. Curr Ornithol 7:251–283
Sichlau MH, Kiørboe T (2011) Age- and size-dependent mating performance and fertility in a pelagic copepod, Temora longicornis. Mar Ecol Prog Ser 442:123–132
Sterner RW, Schulz KL (1998) Zooplankton nutrition: recent progress and a reality check. Aquat Ecol 32:261–279
Thornhill R, Alcock J (1983) The evolution of insect mating systems. Harvard University Press, Harvard
Trivers R (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man. Aldine, Chicago
Turner JT, Ianora A, Miralto A, Laabir M, Esposito F (2001) Decoupling of copepod grazing rates, fecundity and egg-hatching success on mixed and alternating diatom and dinoflagellate diets. Mar Ecol Prog Ser 220:187–199
Uchima M (1985) Copulation in the marine copepod Oithona davisae Ferrari and Orsi. Bull Plankton Soc Jpn 32:31–36
Uye SI, Sano K (1995) Seasonal reproductive biology of the small cyclopoid copepod Oithona davisae in a temperate eutrophic inlet. Mar Ecol Prog Ser 118:121–128
Vahed K (1998) The function of nuptial feeding in insects: a review of empirical studies. Biol Rev Camb Philos Soc 73:43–78
Wedekind C, Jakobsen PJ (1998) Male-biased susceptibility to helminth infection: an experimental test with a copepod. Oikos 81:458–462
Williamson CE, Butler NM (1987) Temperature, food and mate limitation of copepod reproductive rates: separating the effects of multiple hypotheses. J Plankton Res 9:821–836
Acknowledgments
This work was supported by the National Science Foundation (Grant No. OCE-1130284), and a Graduate Research Fellowship from the College of Liberal Arts and Sciences at the University of Connecticut awarded to Z.P. Burris.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by X. Irigoyen.
Rights and permissions
About this article
Cite this article
Burris, Z.P., Dam, H.G. Spermatophore production as a function of food abundance and age in the calanoid copepods, Acartia tonsa and Acartia hudsonica . Mar Biol 162, 841–853 (2015). https://doi.org/10.1007/s00227-015-2628-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-015-2628-6