Abstract
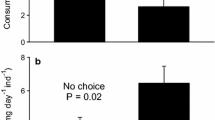
As mean global temperatures continue to rise, regional seawater temperature measurements have revealed that some geographic areas are warming faster than others. One region that is experiencing particularly rapid warming is the western Antarctic Peninsula. Previous studies investigating direct effects of warming on Antarctic marine invertebrates have established that small increases in temperature can have significant impacts on aspects of behavior, physiology, and growth rates in these largely stenothermal organisms. To investigate how warming may impact feeding preferences of an ecologically important mesograzer on macroalgae of the Antarctic Peninsula, we examined the impacts of exposure to acute elevated temperature on the ecologically important omnivorous amphipod Gondogeneiea antarctica (Chevreux) at Palmer Station, Antarctica (64°46′S, 64°03′W) in April–May 2011. Amphipods were exposed to 1.5 °C (mean monthly upper summer temperature) or 3.5 °C (representative of current transient summer temperature peaks and projected mean for 2100) for a 24 h period. These amphipods were then used in choice-feeding assays with artificial food containing chemical extracts from six species of sympatric macroalgae known to produce feeding deterrents. We found that during acute exposure to elevated temperature (+2.0 °C), amphipods lost their feeding preferences in assays with artificial foods containing lipophilic (one macroalga), hydrophilic (three macroalgae), or combined lipophilic/hydrophilic (one macroalga) extracts. Our findings suggest that increased frequency in transient peaks and longer-term upward trends in ambient summer seawater temperature have the potential to alter feeding preferences in a common mesograzer that could influence macroalgal communities that dominate benthic communities along the western Antarctic Peninsula.




Similar content being viewed by others
References
Agardh JG (1848) Species genera et ordines algarum, seu descriptiones succinctae specierum, generum et ordinum, quibus algarum regnum constituitur. Volumen Primum. Algas fucoideas complectens. 2: pp. [i–vi], [i]-viii, [1]-363. C.W.K. Gleerup, Lundae [Lund]
Amsler CD, Iken K, McClintock JB, Amsler MO, Peters KJ, Hubbard JM, Furrow FB, Baker BJ (2005) Comprehensive evaluation of the palatability and chemical defenses of subtidal macroalgae from the Antarctic Peninsula. Mar Ecol Prog Ser 294:141–159
Amsler CD, McClintock JB, Baker BJ (2008) Macroalgal chemical defenses in polar marine communities. In: Amsler CD (ed) Algal chemical ecology. Springer, Berlin, pp 91–103
Amsler MO, Mcclintock JB, Amsler CD, Angus RA, Baker BJ (2009) An evaluation of sponge-associated amphipods from the Antarctic Peninsula. Antarct Sci 21:579–589
Amsler CD, McClintock JB, Baker BJ (2014) Chemical mediation of mutualistic interactions between macroalgae and mesograzers structure unique coastal communities along the western Antarctic Peninsula. J Phycol 50:1–10
Aumack CF, Amsler CD, McClintock JB, Baker BJ (2010) Chemically mediated resistance to mesoherbivory in finely branched macroalgae along the western Antarctic Peninsula. Eur J Phycol 45:19–26
Bindoff NL, Willebrand J, Artale V, Cazenave A, Gregory J, Gulev S, Hanawa K, Le Quéré C, Levitus S, Nojiri Y, Shum CK, Talley LD, Unnikrishnan A (2007) Observations: oceanic climate change and sea level. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Climate change 2007: the physical science basis contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, pp 385–432
Cetrulo GL, Hay ME (2000) Activated chemical defenses in tropical versus temperate seaweeds. Mar Ecol Prog Ser 207:243–253
Clarke A (2001) Benthic organisms and environmental variability in Antarctica: responses to seasonal, decadal and long-term change. Ocean Polar Res 23:433–440
Clarke A, Murphy EJ, Meredith MP, King JC, Peck LS, Barnes DKA, Smith RC (2007) Climate change and the marine ecosystem of the western Antarctic Peninsula. Philos Trans R Soc B 362:149–166
De Broyer C, Jazdzewski K (1996) Biodiversity of the Southern Ocean: towards a new synthesis for the Amphipoda (Crustacea). Bollettino del Museo Civico di Storia Naturale di Verona 20:547–568
De Toni G (1936) Noterelle di nomenclatura algologica. VII. Primo elenco di Floridee omonime. Privately published, Brescia, p 8
Doyle SR, Momo FR, Brêthes JC, Ferreyra GA (2012) Metabolic rate and food availability of the Antarctic amphipod Gondogeneia antarctica (Chevreux 1906): seasonal variation in allometric scaling and temperature dependence. Polar Biol 35:413–424
Ducklow HW, Fraser WR, Meredith MP, Stammerjohn SE, Doney SC, Martinson DG, Sailley SF, Schofield OM, Steinberg DK, Venables HJ, Amsler CD (2013) West Antarctic Peninsula: an ice-dependent coastal marine ecosystem in transition. Oceanography 26:190–203
Fabry VJ, McClintock JB, Mathis JT, Grebmeier JM (2009) Ocean acidification at high latitudes: the bellweather. Oceanography 22:160
Hay ME, Kappel QE, Fenical W (1994) Synergisms in plant defenses against herbivores: interactions of chemistry, calcification, and plant quality. Ecology 75:1714–1726
Huang YM, McClintock JB, Amsler CD, Peters KJ, Baker BJ (2006) Feeding rates of common Antarctic gammarid amphipods on ecologically important sympatric macroalgae. J Exp Mar Biol Ecol 329:55–65
Huang YM, Amsler MO, McClintock JB, Amsler CD, Baker BJ (2007) Patterns of gammaridean amphipod abundance and species composition associated with dominant subtidal macroalgae from the western Antarctic Peninsula. Polar Biol 30:1417–1430
Iken K (1999) Feeding ecology of the Antarctic herbivorous gastropod Laevilacunaria antarctica Martens. J Exp Mar Biol Ecol 236:133–148
Kylin H (1924) Studien über die Delesseriaceen. Acta Univ Lund 20:1–111
Kylin H, Skottsberg C (1919) Zur Kenntnis der subantarktischen und antarktischen Meeresalgen. II. Rhodophyceen. In: Nordenskjöld O (ed) Wissenschaftliche Ergebnisse der Schwedischen Südpolar-Expedition 1901–1903. Litographisches Institut des Generalstabs, Stockholm, pp 1–88
Martinson DG, Stammerjohn SE, Iannuzzi RA, Smith RC, Vernet M (2008) Western Antarctic Peninsula physical oceanography and spatio–temporal variability. Deep-Sea Res Pt II 55:1964–1987
McClintock JB, Baker BJ (2001) Marine chemical ecology. CRC Press, Boca Raton, p 610
Meredith MP, King JC (2005) Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophys Res Lett 32:L19604
Moran MD (2003) Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 100:403–405
O’Connor MI, Gilbert B, Brown CJ (2011) Theoretical predictions for how temperature affects the dynamics of interacting herbivores and plants. Am Nat 178:626–638
O’Connor MI, Piehler MF, Leech DM, Anton A, Bruno JF (2009) Warming and resource availability shift food web structure and metabolism. PLoS ONE 7:e1000178. doi:10.1371/journal.pbio.1000178
Peck L, Morley S, Clark M (2010) Poor acclimation capacities in Antarctic marine ectotherms. Mar Biol 157:2051–2059
Pörtner HO (2002a) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A: Mol Integr Physiol 132:739–761
Pörtner HO (2002b) Physiological basis of temperature-dependent biogeography: trade-offs in muscle design and performance in polar ectotherms. J Exp Biol 205:2217–2230
Pörtner HO, Peck L, Somero G (2007) Thermal limits and adaptation in marine Antarctic ectotherms: an integrative view. Philos Trans R Soc B 362:2233–2258
Skottsberg C (1907) Zur Kenntnis der subantarktischen und antarktischen Meeresalgen. I. Phaeophyceen. Wissenschaftliche Ergebnisse der Schwedischen Südpolar-Expedition 1901–1903 unter Leitung von Dr Otto Nordenskjöld. Lithographisches Institut des Generalstabs, Stockholm, pp 1–172
Sotka EE, Giddens H (2009) Seawater temperature alters feeding discrimination by cold-temperate but not subtropical individuals of an ectothermic herbivore. Biol Bull 216:75–84
Stammerjohn SE, Martinson DG, Smith RC, Iannuzzi RA (2008) Sea ice in the western Antarctic Peninsula region: spatio-temporal variability from ecological and climate change perspectives. Deep-Sea Res Pt II 55:2041–2058
Zinova AD (1959) O dvukh burykh vodoroslyakh iz Antarktiki—Phyllogigas i Himantothallus 1959 [On two brown algae from the Antarctic—Phyllogigas and Himantothallus]. Botanicheski Zhurnal 44:372–379
Acknowledgments
The authors gratefully acknowledge the essential logistical support provided by the staff of Raytheon Polar Services Company at the United States Antarctic Program (USAP). Field collections were aided by the efforts of Margaret Amsler, Kathryn Schoenrock, Bill Dent, and Ruth McDowell. JBS is grateful to Margaret Amsler, Ruth McDowell, and Jacqueline von Salm for training in experimental protocols. We thank Robert A. Angus for his statistical assistance. Palmer Station regional sea surface temperature data were obtained from the Palmer LTER data repository supported by the NSF Office of Polar Programs (NSF Grants OPP-9011927, OPP-9632763 and OPP-0217282). The present study was supported by NSF Grants ANT-0838773 and ANT-1041022 (JBM, CDA) and ANT-0838776 (BJB). Additional support was provided by the UAB Department of Biology and an Endowed Professorship in Polar and Marine Biology to JBM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Weinberger.
Rights and permissions
About this article
Cite this article
Schram, J.B., McClintock, J.B., Amsler, C.D. et al. Impacts of acute elevated seawater temperature on the feeding preferences of an Antarctic amphipod toward chemically deterrent macroalgae. Mar Biol 162, 425–433 (2015). https://doi.org/10.1007/s00227-014-2590-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2590-8