Abstract
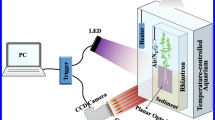
We present a new experimental set-up enabling fine-scale examination of how changing environmental conditions affect the below-ground biogeochemical microenvironment of aquatic macrophytes. By means of microsensor and planar optode technology, the influence of plant-mediated radial O2 release on the below-ground chemical microenvironment of Zostera muelleri and Halophila ovalis was determined in high spatio-temporal resolution. The seagrass specimens were cultured in a new split flow chamber with artificial sediment made of a deoxygenated seawater–agar solution with added sulphide. Microelectrode measurements revealed radial O2 release from the root–shoot junction of both Z. muelleri and H. ovalis during both light stimulation and darkness, resulting in a rapid decrease in H2S concentration, and a significant drop in pH was observed within the plant-derived oxic microzone of Z. muelleri. No radial O2 release was detectable from the below-ground tissue of Z. muelleri during conditions of combined water-column hypoxia and darkness, leaving the plants more susceptible to sulphide invasion. The spatial O2 heterogeneity within the immediate rhizosphere of Z. muelleri was furthermore determined in two dimensions by means of planar optodes. O2 images revealed a decrease in the spatial extent of the plant-derived oxic microzone surrounding the below-ground tissue during darkness, supporting the microelectrode measurements. This new experimental approach can be applied to all rooted aquatic plants, as it allows for direct visual assessment of the below-ground tissue surface during microprofiling, while enabling modification of the above-ground environmental conditions.






Similar content being viewed by others
References
Beer S, Vilenkin B, Weil A, Veste M, Susel L, Eshel A (1998) Measuring photosynthetic rates in seagrasses by pulse amplitude modulated (PAM) fluorometry. Mar Ecol Prog Ser 174:293–300
Binzer T, Borum J, Pedersen O (2005) Flow velocity affects internal oxygen conditions in the seagrass Cymodocea nodosa. Aquat Bot 83:239–247
Borum J et al (2005) The potential role of plant oxygen and sulphide dynamics in die-off events of the tropical seagrass, Thalassia testudinum. J Ecol 93:148–158
Borum J, Sand-Jensen K, Binzer T, Pedersen O, Greve T (2006) Oxygen movements in seagrasses. In: Larkum AWD, Orth JR, Duarte CM (eds) Seagrass Biology. Springer, Dordrecht, pp 255–270
Caffrey JM, Kemp WM (1991) Seasonal and spatial patterns of oxygen production, respiration and root-rhizome release in Potamogeton perfoliatus L. and Zostera marina L. Aquat Bot 40:109–128
Costanza REA (1997) The value of the world’s ecosystem services and natural capital. Nature 387:253–260
Erftemeijer PLA, Lewis RRR (2006) Environmental impacts of dredging on seagrasses: a review. Mar Pollut Bull 52:1553–1572
Frederiksen MS, Glud RN (2006) Oxygen dynamics in the rhizosphere of Zostera marina: a two-dimensional planar optode study. Limnol Oceanogr 51:1072–1083
Glud RN, Ramsing NB, Gundersen JK, Klimat I (1996) Planar optodes: a new tool for fine scale measurements of two-dimensional O2 distribution in benthic communities. Mar Ecol Prog Ser 140:217–226
Greve TM, Borum J, Pedersen O (2003) Meristematic oxygen variability in eelgrass (Zostera marina). Limnol Oceanogr 48:210–216
Holmer M, Bondgaard EJ (2001) Photosynthetic and growth response of eelgrass to low oxygen and high sulfide concentrations during hypoxic events. Aquat Bot 70:29–38
Holmer M et al (2009) Sulfide intrusion in the tropical seagrasses Thalassia testudinum and Syringodium filiforme. Estuar Coast Shelf Sci 85:319–326
Jensen SI, Kühl M, Glud RN, Jorgensen LB, Prieme A (2005) Oxic microzones and radial oxygen loss from roots of Zostera marina. Mar Ecol Prog Ser 293:49–58
Jensen SI, Kühl M, Prieme A (2007) Different bacterial communities in the rhizoplane and bulk sediment of the seagrass Zostera marina. FEMS Microbiol Ecol 62:108–117
Jeroschewski P, Steuckart C, Kühl M (1996) An amperometric microsensor for the determination of H2S in aquatic environments. Anal Chem 68:4351–4357
Kühl M, Revsbech NP (2001) Biogeochemical microsensors for boundary layer studies. In: Boudreau BP, Jorgensen BB (eds) The benthic boundary layer. Oxford University Press, New York, pp 180–210
Larkum AWD, Orth RJ, Duarte CM (2006) Seagrasses: biology, ecology, and conservation. Springer, Berlin
Larsen M, Borisov SM, Grunwald B, Klimant I, Glud RN (2011) A simple and inexpensive high resolution color ratiometric planar optode imaging approach: application to oxygen and pH sensing. Limnol Oceanogr Methods 9:348–360
Orth RJ et al (2006) A global crisis for seagrass ecosystems. Bioscience 56:987–996
Pedersen O, Borum J, Duarte CM, Fortes MD (1998) Oxygen dynamics in the rhizosphere of Cymodocea rotundata. Mar Ecol Prog Ser 169:283–288
Pedersen O, Borum J, Duarte CM, Fortes MD (1999) ERRATUM: oxygen dynamics in the rhizosphere of Cymodocea rotundata. Mar Ecol Prog Ser 178:310
Pedersen O, Binzer T, Borum J (2004) Sulphide intrusion in eelgrass (Zostera marina L.). Plant Cell Environ 27:595–602
Perez-Perez ME, Lemaire SD, Crespo JL (2012) Reactive oxygen species and autophagy in plants and algae. Plant Physiol 160:156–164
Ralph PJ, Short FT (2002) Impact of the wasting disease pathogen, Labyrinthula zosterae, on photobiology of eelgrass Zostera marina. Mar Ecol Prog Ser 226:265–271
Ralph PJ, Tomasko D, Seddon S, Moore K, Macinnis-Ng C (2006) Human impact on seagrasses: contamination and eutrophication in seagrass biology, ecology and conservation. Springer, Berlin, pp 567–593
Raun AL, Borum J (2013) Combined impact of water column oxygen and temperature on internal oxygen status and growth of Zostera marina seedlings and adult shoots. J Exp Mar Biol Ecol 441:16–22
Raven JA, Scrimgeour CM (1997) The influence of anoxia on plants of saline habitates with special reference to the sulfur cycle. Ann Bot 79:79–86
Revsbech NP (1989) An oxygen microsensor with a guard cathode. Limnol Oceanogr 34:474–478
Steen-Knudsen O (2002) Biological membranes: theory of transport, potentials and electric impulses. Cambridge University Press, Cambridge
Waycott M et al (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. PNAS 106:12377–12381
Acknowledgments
We would like to thank technical officer Paul Brooks (UTS) for fruitful discussions and help during the development of the experimental set-up and Frederic Cadera (UTS) for helping with seagrass and sediment sampling. We thank Michael A. Rasheed for his intellectual contribution to the research in question. We acknowledge Ole Pedersen and Jens Borum for thoughtful initial discussions. This research was funded by the Australian Research Council (ARC) (MK, PJR), the Danish Council for Independent Research | Natural Sciences (MK) and through grants from the Augustinus Foundation and Fabrikant P.A. Fiskers Foundation (KEB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Huettel.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brodersen, K.E., Nielsen, D.A., Ralph, P.J. et al. A split flow chamber with artificial sediment to examine the below-ground microenvironment of aquatic macrophytes. Mar Biol 161, 2921–2930 (2014). https://doi.org/10.1007/s00227-014-2542-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2542-3