Abstract
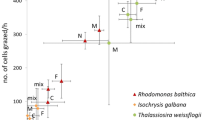
Meroplankton are seasonally important contributors to the zooplankton, particularly at inshore sites, yet their feeding ecology is poorly known relative to holoplankton. While several studies have measured feeding in decapod larvae, few studies have examined the feeding rates of decapod larvae on natural prey assemblages throughout the reproductive season. We conducted 8 feeding experiments with Necora puber, Liocarcinus spp. and Upogebia spp. zoea larvae collected from the L4 monitoring site off Plymouth (50°15.00′N, 4°13.02′W) during spring–summer 2009 and 2010. This period spanned moderate-to-high food availability (0.5–1.6 µg chl-a L−1), but a great range in food composition with small cells <20 µm dominating in 2010. Daily rations averaged 17, 60 and 22 % of body C for the 3 respective decapod species. Clearance rates differed according to prey type, and all 3 decapod genera showed evidence of selection of dinoflagellates. Importantly, small cells including nano- and pico-plankton were ingested, this being demonstrated independently by flow cytometric analysis of the feeding experiments and molecular analysis. PCR-based analysis of the haptophyte portion of the diet revealed ingestion of Isochrysis galbana by decapod larvae in the bottle incubations and Isochrysis galbana and Phaeocystis globosa by decapod larvae collected directly from the field. This study has shown that pico- and nano-sized plankton form an important supplement to the diverse and variable diet of decapod larvae.







Similar content being viewed by others
References
Almeda R, Messmer AM, Sampedro N, Gosselin LA (2010) Feeding rates and abundance of marine invertebrate planktonic larvae under harmful algal bloom conditions off Vancouver Island. Harmful Algae 10:194–206
Anger K (1990) Modelling developmental changes in the carbon and nitrogen budgets of larval brachyuran crabs. Helgol Meeresunters 44:53–80
Anger K (2001) The biology of Decapod Crustacean Larvae. In: Vonk R (ed) Crustacean issues 14 A.A. Balkema, Lisse
Anger K, Dawirs RR (1981) Influence of starvation on the larval development of Hyas araneus (Decapoda, Majidae). Helgol Wiss Meeresunters 34:287–311
Anger K, Dawirs RR, Anger RV, Costlow JD (1981) Effects of early starvation periods on zoeal development of brachyuran crabs. Biol Bull 161:199–212
Atkinson A (1995) Omnivory and feeding selectivity in five copepod species during spring in the Bellingshausen Sea, Antarctica. ICES J Mar Sci 52:385–396
Atkinson A, Fileman ES, Widdicombe C, Harmer R, McEvoy A, Harris RP, Smyth T (2013) Plymouth L4 (Site 48) In: O’Brien TD, Wiebe PH, Falkenhaug T (eds) ICES Zooplankton Status Report 2010/2011. ICES Cooperative Research Report No. 318, pp 127–131
Azam F, Fenchel T, Field JG, Gray JS, Meter-Reil LA, Thingstad F (1983) The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10:257–263
Barnes MK, Tilstone G, Smyth T, Widdicombe CE, Glöel J, Robinson C, Suggett DJ (2014) Drivers and effects of Karenia mikimotoi blooms in the western English Channel. Prog Oceanog (in review)
Bigelow HB, Lillick LC, Sears M (1940) Phytoplankton and planktonic protozoa of the offshore waters of the Gulf of Maine. Trans Am Phil Soc 31:149–237
Boidron-Métairon IF (1995) Larval nutrition. In: McEdward L (ed) Ecology of marine invertebrate larvae. CRC Press, New York, pp 223–248
Booth BC, Lewin J, Lorenzen CJ (1988) Spring and summer growth rates of subarctic Pacific phytoplankton assemblages determined from carbon uptake and cell volumes estimated using epifluorescence microscopy. Mar Biol 98:287–298
Burnet N, Sulkin S (2007) Characteristics of feeding on dinoflagellates by newly hatched larval crabs. Mar Biol 151:851–861
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Tumbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Chen X, Lin Q, Wang G, Li S (2013) Feeding in the megalopae of the mud crab (Scylla paramamosain): mechanisms, plasticity, role of chelipeds and effect of prey density. Mar Fresh Behav Phys 46:321–336
Choy SC (1991) Embryonic and larval biology of Liocarcinus holsatus and Necora puber (Crustacea: Brachyura: Portunidae). J Exp Mar Biol Ecol 148:77–92
Coolen MJL, Muyzer G, Rijpstra WIC, Schouten S, Volkman JK, Damste JSS (2004) Combined DNA and lipid analyses of sediments reveal changes in Holocene haptophyte and diatom populations in an Antarctic lake. Earth Planet Sc Lett 223:225–239
Durbin EG, Casas MG, Rynearson TA (2012) Copepod feeding and digestion rates using prey DNA and qPCR. J Plankton Res 34:72–82
Edwards M, Richardson AJ (2004) Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430:881–884
Eloire D, Somerfield PJ, Conway DVP, Halsband-Lenk C, Harris R, Bonnet D (2010) Temporal variability and community composition of zooplankton at station L4 in the Western Channel: 20 years of sampling. J Plankton Res 32:657–679
Epifanio CE, Lobanoff MA, Connaughton VP, Welch JM (1994) Growth and development of Atlantic mud crab larvae fed natural zooplankton prey. J Exp Mar Biol Ecol 180:165–174
Factor JR, Dexter BL (1993) Suspension feeding in larval crabs (Carcinus maenas). J Mar Biol Assoc UK 73:207–211
Fileman E, Petropavlovsky A, Harris R (2010) Grazing by the copepods Calanus helgolandicus and Acartia clausi on the protozooplankton community station L4 in the Western English Channel. J Plankton Res 32:709–724
Fileman ES, Fitzgeorge-Balfour T, Tarran GA, Harris RP (2011) Plankton community diversity from bacteria to copepods in bloom and non-bloom conditions in the Celtic Sea in spring. Estuar Coast Shelf Sci 93:403–414
Frost BW (1972) Effect of size and concentration of food particles on the feeding behaviour of the marine planktonic copepod Calanus pacificus. Limnol Oceanogr 17:805–815
Harms J, Seeger B (1989) Larval development and survival in seven decapod species (Crustacea) in relation to laboratory diet. J Exp Mar Biol Ecol 133:129–139
Harms J, Meyer-Harms B, Dawirs RR, Anger K (1994) Growth and physiology of Carcinus maenas (Decapoda: Portunidae) larvae in the field and in laboratory experiments. Mar Ecol Prog Ser 108:107–118
Hartman MC, Letterman GR (1978) An evaluation of three species of diatoms as food for Cancer magister larvae. In: Proceedings 9th annual meeting world mariculture society, pp 271–276
Harvey EA, Epifanio CE (1997) Prey selection by larvae of the common mud crab Panopeus herbstii Milne-Edwards. J Exp Mar Biol Ecol 217:79–91
Harvey M, Morrier G (2003) Laboratory feeding experiments on zoea of northern shrimp Pandalus borealis fed with natural zooplankton. Mar Ecol Prog Ser 265:165–174
Heidecker G, Messing J, Gronenborn B (1980) A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene 10:69–73
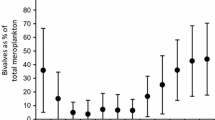
Highfield JM, Eloire D, Conway D, Lindeque P, Attrill MJ, Somerfield PJ (2010) Seasonal dynamics of meroplankton assemblages at station L4. J Plankton Res 32:681–691
Hinz S, Sulkin S, Strom S, Testermann J (2001) Discrimination in ingestion of protistan prey by larval crabs. Mar Ecol Prog Ser 222:155–162
Holthuis LB (1987) Necora, a new genus of European swimming crabs (Crustacea Decapoda, Portunidae) and its type species, Cancer puber L., 1767. Zoologische Mededelingen 61:1–14
Incze LS, Paul AJ (1983) Grazing and predation as related to energy needs of stage I zoeae of the tanner crab Chionoecefes bairdi (Brachyura, Majidae). Biol Bull (Woods Hole) 165:197–220
Irigoien X, Harris RP, Head RN, Harbour D (2000a) North Atlantic Oscillation and spring bloom phytoplankton composition in the English Channel. J Plankton Res 22:2367–2371
Irigoien X, Head RN, Harris RP, Cummings D, Harbour D, Meyer-Harms B (2000b) Feeding selectivity and egg production of Calanus helgolandicus in the English Channel. Limnol Oceanogr 45:44–54
Irigoien X, Flynn KJ, Harris RP (2005) Phytoplankton blooms: a “loophole” in microzooplankton grazing impact? J Plankton Res 27:313–321
Johnson PR, Tiselius P (1990) Feeding behaviour, prey detection and capture efficiency of the copepods Acartia tonsa feeding on planktonic ciliates. Mar Ecol Prog Ser 60:35–44
Kiørboe T (2000) Colonization of marine snow aggregates by invertebrate zooplankton: abundance, scaling, and possible role. Limnol Oceanogr 45:479–484
Kiørboe T (2011) How zooplankton feed: mechanisms, traits and trade-offs. Biol Rev 86:311–339
Kovala PE, Larrance JD (1966) Computation of phytoplankton cell numbers, cell volume, cell surface area and plasma volume per litre, from microscopical counts. Special Report, vol. 38. University of Washington, Seattle, pp 1–91
Lebour M (1922) The food of plankton organisms. J Mar Biol Assoc UK 12:644–677
Lehto J, Sulkin SD, Strom S, Johnson D (1998) Protists and detrital particles as prey for the first larval stage of the brachyuran crab, Hemigrapsus oregonensis. J Exp Mar Biol Ecol 230:213–224
Lindeque PK, Parry HE, Harmer RA, Somerfield PJ, Atkinson A (2013) Next generation sequencing reveals the hidden diversity of zooplankton assemblages. PLoS ONE 8(11):e81327
Lindeque PK, Dimond A, Harmer RA, Parry HE, Pemberton K, Fileman ES (2014) Feeding selectivity of bivalve larvae on natural plankton assemblages in the Western English Channel. Mar Biol (in review)
Lindley JA (1987) Continuous plankton records: the geographical distribution and seasonal cycles of decapod crustacean larvae and pelagic post-larvae in the north-eastern Atlantic Ocean and the North Sea. J Mar Biol Assoc UK 122:195–211
Lindley JA (1998) Diversity, biomass and production of decapod crustacean larvae in a changing environment. Invertebr Reprod Dev 33:209–219
Lindley JA, Williams R, Conway DVP (1994) Variability in dry weight and vertical distributions of decapod larvae in the Irish Sea and North Sea during the spring. Mar Biol 120:395
McConaugha JR (2002) Alternative feeding mechanisms and prey selection in post-larval megalopae of the blue crab, Callinectes sapidus. Mar Biol 140:1227–1233
Menden-Deuer S, Lessard EJ (2000) Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol Oceanogr 45:569–579
Meyer-Harms B, Harms J (1993) Detection of phytoplankton pigments by HPLC in Hyas araneus larvae (crustacea, decapoda): comparison of field and laboratory samples. Neth J Sea Res 31:153–161
Montagnes DJS, Berges JA, Harrison PA, Taylor FJR (1994) Estimating carbon, nitrogen, protein and chlorophyll a from volume in marine phytoplankton. Limnol Oceanogr 39:1044–1060
Nejstgaard JC, Frischer ME, Raule CL, Gruebel T, Kohlberg KE, Verity PG (2003) A new approach to an old problem: molecular detection of algal prey in copepod guts and faecal pellets. Limnol Oceanogr Method 1:29–38
Nejstgaard JC, Frischer ME, Simonelli P, Troedsson C, Brakel M, Adiyaman F, Sazhin AF, Artigas AF (2008) Quantitative PCR to estimate copepod feeding. Mar Biol 153:565–577
Olson RR, Olson MH (1989) Food limitation of planktotrophic marine invertebrate larvae: does it control recruitment success? Ann Rev Ecol Syst 20:225–247
Pan M, Pierce GJ, Cunningham CO, Hay SJ (2011) Seasonal and interannual variation of decapod larval abundance from two coastal locations in Scotland, UK. J Mar Biol Ass UK 91:1443–1451
Perez MF, Sulkin SD (2005) Palatability of autotrophic dinoflagellates to newly hatched larval crabs. Mar Biol 146:771–780
Pingree RD, Griffiths DK (1978) Tidal fronts on the shelf seas around the British Isles. J Geophys Res 83:4615–4622
Price HJ, Paffenhöfer G-A (1983) Capture of small cells by the copepod Eucalanus elongatus. Limnol Oceanogr 31:189–194
Roman MR, Rublee PA (1980) Containment effects in copepod grazing experiments: a plea to end the black box approach. Limnol Oceanogr 25:982–990
Schwamborn R, Ekau W, Silva AP, Neumann-Leitào S, Saint-Paul U (2006) Ingestion of large centric diatoms, mangrove detritus, and zooplankton by zoeae of Aratus pisonii (Crustacea: Brachyura: Grapsidaae). Hydrobiologia 560:1–13
Shaber K, Sulkin S (2007) Feeding on dinoflagellates by intermediate and late stage crab zoeae raised in the laboratory and collected from the field J Exp Mar Biol Ecol 340:149–159
Simon N, Campbell L, Ornolfsdottir E, Groben R, Guillou L, Lange M, Medlin LK (2000) Oligonucleotide probes for the identification of three algal groups by dot blot and fluorescent whole-cell hybridization. J Eukaryot Microbiol 47:76–84
Smyth T, Fishwick J, Al-Moosawi L, Cummings DG, Harris C, Kitidis V, Rees A, Martinez-Vicente V, Woodward EMS (2010) A broad spatio-temporal view of the Western English Channel observatory. J Plankton Res 32:585–601
Strathmann RR (1987) Larval Feeding. In: Giese AC, Pearse JS, Pearse VB (eds) Reproduction of marine invertabrates. General aspects: seeking unity in diversity, vol 9. Blackwell Scientifica Publications, Palo Alto, Californie, USA, pp 465–550
Strathmann RR (1995) Are planktonic larvae of marine benthic invertebrates too scarce to compete within species ? Oceanol Acta 19:399–407
Sulkin SD, Lehto J, Strom S, Hutchinson D (1998) Nutritional role of protists in the diet of first stage larvae of the Dungeness crab Cancer magister. Mar Ecol Prog Ser 169:237–242
Tarran GA, Heywood JL, Zubkov MV (2006) Latitudinal changes in the standing stocks of nano- and picoeukaryotic phytoplankton in the Atlantic Ocean. Deep-Sea Res II 53:1516–1529
Thorson G (1946) Reproduction and larval development of Danish Marine bottom invertebrates. Medd Komm Dan Fisk-Havunders Ser Plankton 4:1–523
Utermöhl H (1958) Zur vervollkommnung der quantitativen phytoplankton methodik. Mitteilungen der Internationalen Vereinigung für Theoretische und Angewandte Limnologie 9:1–38
Vanderploeg HA, Scavia D (1979) Calculation and use of selectivity coefficients of feeding: zooplankton grazing. Ecol Model 7:135–149
Vargas CA, Manriquez P, Navarrette SA (2006) Feeding by larvae of intertidal invertebrates: assessing their position in pelagic trophic webs. Ecology 87:444–457
Welch JM, Epifanio CE (1995) Effect of variations in prey abundance on growth and development of crab larvae reared in the laboratory and in large, field-deployed enclosures. Mar Ecol Prog Ser 116:55–64
Widdicombe, CE Eloire D, Harbour D, Harris R, Somerfield PJ (2010a) Time series of phyto- and microzooplankton abundance and composition at station L4 in the English Channel from 1988 to 2009. doi:10.1594/PANGAEA.758061
Widdicombe C, Eloire D, Harbour D, Harris R, Somerfield PJ (2010b) Long-term phytoplankton community dynamics in the Western English Channel. J Plankton Res 32:643–655
Wiltshire KH, Malzahn AM, Wirtz K, Greve W, Janisch S, Mangelsdorf P, Manly B, Boersma M (2008) Resilience of North Sea phytoplankton spring bloom dynamics: an analysis of long term data at Helgoland Roads. Limnol Oceanogr 53:1294–1302
Wirtz KW (2012) Who is eating whom? Morphology and feeding type determine the size relation between planktonic predators and their ideal prey. Mar Ecol Prog Ser 445:1–12
Acknowledgments
This work was in part funded by the EU INTERREG IV CHARM 3 project (www.charm-project.org) (Management number 4037/1938) and the Natural Environment Research Council National Capability funding for the Western Channel Observatory (Agreement number R8-H12-84). We are grateful to Denise Cummings and the crew of PML RV Quest for water collection, James Highfield for experimental help, Katharine Pemberton for molecular analysis, Glen Tarran for help and advice on flow cytometry, Claire Widdicombe for L4 phytoplankton data, Denise Cummings for L4 chlorophyll data and John Bruun for his expert statistical advice. We also thank three anonymous reviewers whose comments have greatly improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by X. Irigoyen.
Rights and permissions
About this article
Cite this article
Fileman, E.S., Lindeque, P.K., Harmer, R.A. et al. Feeding rates and prey selectivity of planktonic decapod larvae in the Western English Channel. Mar Biol 161, 2479–2494 (2014). https://doi.org/10.1007/s00227-014-2520-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2520-9