Abstract
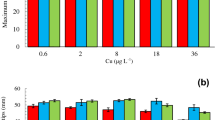
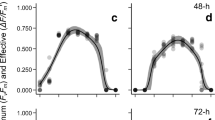
The combined effects of exposure to copper and temperature were investigated in adult specimens and germlings of the canopy-forming brown alga Fucus serratus. A matrix of four temperatures, 6, 12, 17 and 22 °C, and three concentrations of copper, 0, 100 and 1,000 nM total copper were used. Measured endpoints were growth rate, chlorophyll fluorescence parameters and for germlings also survival. The growth rate of adult specimens of F. serratus changed with increasing temperature. Growth tended to be negatively affected by high concentrations of copper when exposed to heat (22 °C) though not significantly so. The photosynthetic performance (i.e., chlorophyll fluorescence parameters: F v/F m, maximum electron transport rate (ETRmax) and maximum non-photosynthetic quenching (NPQmax) of adults was largely unaffected by both copper and temperature. Germling survival, growth rate and chlorophyll fluorescence parameters were affected by the combination of copper concentration and temperature. Increasing temperature led to reduced survival, increased rhizoid growth and higher F v/F m and ETRmax, whereas high copper concentration had a negative effect on the latter three endpoints. The negative effect of high copper concentration was amplified by high temperature. We conclude that juveniles of F. serratus are more susceptible to environmental stressors than adult specimens and recommend therefore including early life stages when assessing the risk of exposure to toxic compounds. Considering the response of adult specimens only may lead to false conclusions regarding the ecological impact of environmental stress.





Similar content being viewed by others
References
Adey WH, Steneck RS (2001) Thermogeography over time creates biogeographic regions: a temperature/space/time-integrated model and an abundance-weighted test for benthic marine algae. J Phycol 37:677–698
Altamirano M, Flores-Moya A, Figueroa FL (2003) Effects of UV radiation and temperature on growth of germlings of three species of Fucus (Phaeophyceae). Aquat Bot 75:9–20
Andersen GS, Pedersen MF, Nielsen SL (2013) Temperature acclimation and heat tolerance of photosynthesis in Norwegian Saccharina latissima (Laminariales, Phaeophyceae). J Phycol 49:689–700
Anderson WH, Gorley RN, Clarke KR (2008) Permanova + for Primer. Guide to software and statistical methods. PRIMER-E Ltd., Plymouth
Andersson S, Kautsky L (1996) Copper effects on reproductive stages of Baltic Sea fucus vesiculosus. Mar Biol 125:171–176
Baumann HA, Morrison L, Stengel DB (2009) Metal accumulation and toxicity measured by PAM-chlorophyll fluorescence in seven species of marine macroalgae. Ecotoxicol Environ Saf 72:1063–1075
Begin C, Johnson LE, Himmelman JH (2004) Macroalgal canopies: distribution and diversity of associated invertebrates and effects on the recruitment and growth of mussels. Mar Ecol Prog Ser 271:121–132
Bond PR, Brown MT, Moate RM, Gledhill M, Hill SJ, Nimmo M (1999) Arrested development in Fucus spiralis (Phaeophyceae) germlings exposed to copper. Eur J Phycol 34:513–521
Brownlee C, Bouget FY (1998) Polarity determination in fucus: from zygote to multicellular embryo. Semin Cell Dev Biol 9:179–185
Connan S, Stengel DB (2011) Impacts of ambient salinity and copper on brown algae: 1. Interactive effects on photosynthesis, growth, and copper accumulation. Aquat Toxicol 104:94–107
Dethier MN, Williams SL (2009) Seasonal stresses shift optimal intertidal algal habitats. Mar Biol 156:555–567
Diez I, Muguerza N, Santolaria A, Ganzedo U, Gorostiaga JM (2012) Seaweed assemblage changes in the eastern Cantabrian Sea and their potential relationship to climate change. Estuar Coast Shelf Sci 99:108–120
Eklund BT, Kautsky L (2003) Review on toxicity testing with marine macroalgae and the need for method standardization—exemplified with copper and phenol. Mar Pollut Bull 46:171–181
Fredersdorf J, Muller R, Becker S, Wiencke C, Bischof K (2009) Interactive effects of radiation, temperature and salinity on different life history stages of the Arctic kelp Alaria esculenta (Phaeophyceae). Oecologia 160:483–492
Harley CDG, Anderson KM, Demes KW, Jorve JP, Kordas RL, Coyle TA, Graham MH (2012) Effects of climate change on global seaweed communities. J Phycol 48:1064–1078
Kevekordes K (2000) The effects of secondary-treated sewage effluent and reduced salinity on specific events in the early life stages of Hormosira banksii (Phaeophyceae). Eur J Phycol 35:365–371
Kevekordes K, Clayton MN (2000) Development of Hormosira banksii (Phaeophyceae) embryos in selected components of secondarily-treated sewage effluent. J Phycol 36:25–32
Knight M, Parke M (1950) A biological study of Fucus vesiculosus and F. serratus. J Mar Biol Assoc UK 29:87–90
La Rocca N, Andreoli C, Giacometti GM, Rascio N, Moro I (2009) Responses of the Antarctic microalga Koliella antarctica (Trebouxiophyceae, Chlorophyta) to cadmium contamination. Photosynthetica 47:471–479
Larcher W (2001) Physiological plant ecology. Ecophysiology and stress physiology of functional groups. Springer, Berlin
Lüning K (1984) Temperature tolerance and biogeography of seaweeds—the marine algal flora of Helgoland (North Sea) as an example. Helgolander Meeresuntersuchungen 38:305–317
Malm T, Kautsky L, Engkvist R (2001) Reproduction, recruitment and geographical distribution of Fucus serratus L. in the Baltic Sea. Bot Mar 44:101–108
Martinez B, Arenas F, Rubal M, Burgues S, Esteban R, Garcia-Plazaola I, Figueroa FL, Pereira R, Saldana L, Sousa-Pinto I, Trilla A, Viejo RM (2012) Physical factors driving intertidal macroalgae distribution: physiological stress of a dominant fucoid at its southern limit. Oecologia 170:341–353
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Morel FMM, Rueter JG, Anderson DM, Guillard RRL (1979) Aquil: a chemically defined phytoplankton culture medium for trace metal studies. J Phycol 15:135–141
Muller R, Laepple T, Bartsch I, Wiencke C (2009) Impact of oceanic warming on the distribution of seaweeds in polar and cold-temperate waters. Bot Mar 52:617–638
Nielsen HD, Nielsen SL (2005) Photosynthetic responses to Cu2+ exposure are independent of light acclimation and uncoupled from growth inhibition in Fucus serratus (Phaeophyceae). Mar Pollut Bull 51:715–721
Nielsen HD, Nielsen SL (2008) Evaluation of imaging and conventional PAM as a measure of photosynthesis in thin- and thick-leaved marine macroalgae. Aquat Biol 3:121–131
Nielsen HD, Nielsen SL (2010) Adaptation to high light irradiances enhances the photosynthetic Cu2+ resistance in Cu2+ tolerant and non-tolerant populations of the brown macroalgae Fucus serratus. Mar Pollut Bull 60:710–717
Nielsen HD, Brown MT, Brownlee C (2003a) Cellular responses of developing Fucus serratus embryos exposed to elevated concentrations of Cu2+. Plant Cell Environ 26:1737–1747
Nielsen HD, Brownlee C, Coelho SM, Brown MT (2003b) Inter-population differences in inherited copper tolerance involve photosynthetic adaptation and exclusion mechanisms in Fucus serratus. New Phytol 160:157–165
Nielsen HD, Burridge TR, Brownlee C, Brown MT (2005) Prior exposure to Cu contamination influences the outcome of toxicological testing of Fucus serratus embryos. Mar Pollut Bull 50:1675–1680
Nygard CA, Dring MJ (2008) Influence of salinity, temperature, dissolved inorganic carbon and nutrient concentration on the photosynthesis and growth of Fucus vesiculosus from the Baltic and Irish Seas. Eur J Phycol 43:253–262
Pohl C, Hennings U (1999) Bericht zum Ostsee-Monitoring: die Schwermetall-Situation in der Ostsee im Jahre 1999. Institut für Ostseeforschung
Pohl C, Kattner G, Schulzbaldes M (1993) Cadmium, copper, lead and zinc on transects through arctic and eastern Atlantic surface and deep waters. J Mar Syst 4:17–29
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Roberts SK, Berger F, Brownlee C (1993) The role of Ca2+ in signal-transduction following fertilization in Fucus serratus. J Exp Biol 184:197–212
Steen H (2004a) Effects of reduced salinity on reproduction and germling development in Sargassum muticum (Phaeophyceae, Fucales). Eur J Phycol 39:293–299
Steen H (2004b) Interspecific competition between Enteromorpha (Ulvales: chlorophyceae) and Fucus (Fucales : phaeophyceae) germlings: effects of nutrient concentration, temperature, and settlement density. Mar Ecol Prog Ser 278:89–101
Steen H, Rueness J (2004) Comparison of survival and growth in germlings of six fucoid species (fucales, Phaeophyceae) at two different temperature and nutrient levels. Sarsia 89:175–183
Steen H, Scrosati R (2004) Intraspecific competition in Fucus serratus and F. evanescens (phaeophyceae : fucales) germlings: effects of settlement density, nutrient concentration, and temperature. Mar Biol 144:61–70
Suzuki N, Mittler R (2006) Reactive oxygen species and temperature stresses: a delicate balance between signaling and destruction. Physiol Plant 126:45–51
Wernberg T, Russell BD, Thomsen MS, Gurgel CFD, Bradshaw CJA, Poloczanska ES, Connell SD (2011) Seaweed communities in retreat from Ocean warming. Curr Biol 21:1828–1832
Wiencke C, Bartsch I, Bischoff B, Peters AF, Breeman AM (1994) Temperature requirements and biogeography of antarctic, arctic and amphiequatorial seaweeds. Bot Mar 37:247–259
Xylander M, Fischer W, Braune W (1998) Influence of mercury on the green alga Haemotococcus lacustris. Inhibition effects and recovery of impact. Botanica Acta 111:467–473
Zou DH, Liu SX, Du H, Xu JT (2012) Growth and photosynthesis in seedlings of Hizikia fusiformis (Harvey) Okamura (Sargassaceae, Phaeophyta) cultured at two different temperatures. J Appl Phycol 24:1321–1327
Acknowledgments
HDN was supported by a grant from the Danish Natural Science Research Council. The council had no involvement in study design, data collection and interpretation, or writing and submitting the paper. We thank Theresa Fernandes and Paul Tett at Napier University, Edinburgh, for housing and supporting HDN during work on this project. We thank Dr. Gary T. Banta for advice on statistical methods.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Weinberger.
Rights and permissions
About this article
Cite this article
Nielsen, S.L., Nielsen, H.D. & Pedersen, M.F. Juvenile life stages of the brown alga Fucus serratus L. are more sensitive to combined stress from high copper concentration and temperature than adults. Mar Biol 161, 1895–1904 (2014). https://doi.org/10.1007/s00227-014-2471-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2471-1