Abstract
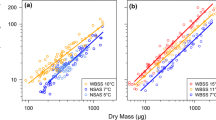
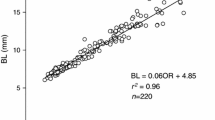
Juvenile oysters (Crassostrea gigas) (produced in November 2009) reared under uniform hatchery conditions for 4 months were selected for extreme growth rate differences by repeatedly taking larger and smaller individuals to achieve weight differences >30× between fast (F) and slow (S) growers. The physiological basis of differential growth was analyzed in experiments in June 2010, where components of energy gain (clearance and ingestion rates and absorption efficiency), energy loss (metabolic rates) and resulting scope for growth (J h−1) were compared for groups of F and S oysters fed three different ration levels (≈0.5, 1.5 and 3.0 mg of total particulate matter L−1). In both F and S oysters, a higher food ration promoted asymptotic increases in energy gain rates through regulatory adjustments to clearance rates, which maintained similar absorption efficiencies across the food concentrations. No significant differences were found between growth groups in mass-specific physiological rates (i.e., per unit of body mass). However, the scaling of these rates to a common size in both groups using allometric coefficients derived for C. gigas revealed higher energy gain rates coupled with lower metabolic costs of growth in fast growers. Thus, appropriate size-standardization is essential in accounting for observed differences in growth rate. Present results are in accordance with previous reports on other bivalve species on the physiological processes underlying endogenous growth differences, suggesting that the same interpretation can be applied to the extremes of these differences.




Similar content being viewed by others
References
Barillé L, Prou J, Héral M, Razet D (1997) Effects of high natural seston concentrations on the feeding, selection, and absorption of the oyster Crassostrea gigas (Thunberg). J Exp Mar Biol Ecol 212:149–172
Batista F, Leitão A, Fonseca V, Ben-Hamadou R, Ruano F, Henriques M, Guedes-Pinto H, Boudry P (2007) Individual relationship between aneuploidy of gill cells and growth rate in cupped oysters Crassostrea angulata, C. gigas and their reciprocal hybrids. J Exp Mar Biol Ecol 352:226–233
Bayne BL (1999) Physiological components of growth differences between individual oysters (Crassostrea gigas) and a comparison with Saccostrea commercialis. Physiol Biochem Zool 72(6):705–713
Bayne BL (2000) Relations between variable rates of growth, metabolic costs and growth efficiencies in individual Sydney rock oysters (Saccostrea commercialis). J Exp Mar Biol Ecol 251:185–203
Bayne BL (2004) Phenotypic flexibility and physiological tradeoffs in the feeding and growth of marine bivalve molluscs. Integr Comp Biol 44:425–432
Bayne BL, Hawkins AJS (1997) Protein metabolism, the costs of growth, and genomic heterozygosity: experiments with the mussel Mytilus galloprovincialis Lmk. Physiol Zool 70:391–402
Bayne BL, Newell RC (1983) Physiological energetics of marine molluscs. In: Salenium ASM, Wilbur KM (eds) The mollusca, Academic Press, New York, 4(1) pp 407–515
Bayne BL, Hedgecock D, McGoldrick D, Rees R (1999a) Feeding behaviour and metabolic efficiency contribute to growth heterosis in Pacific oysters [Crassostrea gigas (Thunberg)]. J Exp Mar Biol Ecol 233:115–130
Bayne BL, Svensson S, Nell JA (1999b) The physiological basis for faster growth in the Sydney rock oyster, Saccostrea commercialis. Biol Bull 197:377–387
Bougrier S, Geairon P, Deslous-Paoli JM, Bacher C, Jonquières G (1995) Allometric relationships and effects of temperature on clearance and oxygen consumption rates of Crassostrea gigas (Thunberg). Aquaculture 134:143–154
Brown JR (1988) Multivariate analyses of the role of environmental factors and seasonal and site-related growth variation in the Pacific oyster, Crassostrea gigas. Mar Ecol Progr Ser 45:225–236
Conover RJ (1966) Assimilation of organic matter by zooplankton. Limnol Oceanogr 11:338–354
Crisp DJ (1971) Energy flow measurements. In: Holme NA, Mc Intyre AD (eds) Methods for the study of marine benthos, (IBP nº 16). Blackwell, Oxford, p 197–323
Dickie LM, Boudreau PR, Freeman KR (1984) Influence of stock and site on growth and mortality in the blue mussel (Mytilus edulis). Can J Fish Aquat Sci 41:134–140
Evans S, Langdon C (2006) Direct and indirect responses to selection on individual body weight in the pacific oyster (Crassostrea gigas). Aquaculture 261:546–555
Gaffney PM, Scott TM, Koehn RK, Diehl WJ (1990) Interrelationships of heterozygosity, growth rate and heterozygote deficiencies in the coot clam, Munilia lateralis. Genetics 124:687–699
Garton DW, Koehn RK, Scott TM (1984) Multiple-locus heterozygosity and the physiological energetics of growth in the coot clam, Mulinia lateralis, from a natural population. Genetics 108:445–455
Genard B, Miner P, Nicolas J-L, Moraga D, Boudry P, Pernet F, Tremblay R (2013) Integrative study of physiological changes associated with bacterial infection in Pacific oyster larvae. PLoS One 8(5):e64534. doi:10.1371/journal.pone.0064534
Gnaiger E (1983) Heat dissipation and energetic efficiency in animal anoxibiosis: economy contra power. J Exp Zool 228:471–490
Haure J, Huvet A, Palvadeau H, Nourry M, Penisson C, Martin JLY, Boudry P (2003) Feeding and respiratory time activities in the cupped oysters Crassostrea gigas, Crassostrea angulata and their hybrids. Aquaculture 218:539–551
Hawkins AJS (1995) Effects of temperature change on ectotherm metabolism and evolution: metabolic and physiological interrelations underlying the superiority of multi-locus heterozygotes in heterogeneous environments. J Therm Biol 2:23–33
Hawkins AJS, Day AJ (1996) The metabolic basis of genetic differences in growth efficiency among marine animals. J Exp Mar Biol Ecol 203:93–115
Hawkins AJS, Bayne BL, Day AJ (1986) Protein turnover, physiological energetics and heterozygosity in the blue mussel Mytilus edulis: the basis of variable age-specific growth. Proc R Soc Lond 229B:161–176
Hawkins AJS, Widdows J, Bayne BL (1989a) The relevance of whole-body protein metabolism to measured costs of maintenance and growth in Mytilus edulis. Physiol Zool 62:745–763
Hawkins AJS, Bayne BL, Day AJ, Rusin J, Worrall CM (1989b) Genotype-dependent interrelations between energy metabolism, protein metabolism and fitness. In: Ryland JS, Tyler PA (eds) Reproduction, genetics and distribution of marine organisms. Olsen and Olsen, Fredensborg, pp 283–292
Hedgecock D, McGoldrick DJ, Manahan DT, Vavra J, Appelmans N, Bayne BL (1995) Quantitative and molecular genetic analyses of heterosis in bivalve molluscs. J Exp Mar Biol Ecol 203:49–59
Hedgecock D, Lin JZ, DeCola S, Haudenschild CD, Meyer E, Manahan DT, Bowen B (2007) Transcriptomic analysis of growth heterosis in larval Pacific oysters (Crassostrea gigas). Proc Natl Acad Sci USA 104:2313–2318
Ibarrola I, Larretxea X, Navarro E, Iglesias JIP, Urrutia MB (2008) Effects of body-size and season on digestive organ size and the energy balance of cockles fed with a constant diet of phytoplankton. J Comp Physiol B 178:501–514
Ibarrola I, Arambalza U, Navarro JM, Urrutia MB, Navarro E (2012) Allometric relationships in feeding and digestion in the Chilean mytilids Mytilus chilensis (Hupé), Choromytilus chorus (Molina) and Aulacomya ater (Molina): a comparative study. J Exp Mar Biol Ecol 426:18–27
Jenny MJ, Chapman RW, Annalaura M et al (2007) A cDNA microarray for Crassostrea virginica and C. gigas. Mar Biotech 9:577–591
Koehn RK, Gaffney PM (1984) Genetic heterozygosity and growth rate in Mytilus edulis. Mar Biol 82:1–7
Launey S, Hedegecock D (2001) High genetic load in the Pacific oyster Crassostrea gigas. Genetics 159:255–265
Leitão A, Boudry P, Thiriot-Quiévreux C (2001) Negative correlation between aneuploidy and growth in the Pacific oyster Crassostrea gigas: ten years of evidence. Aquaculture 193:39–48
Mallet AL, Haley LH (1983) Growth rate and survival in pure population mating and crosses of the oyster Crassostrea virginica. Can J Fish Aquat Sci 40:948–954
Meyer E, Manahan DT (2010) Gene expression profiling of genetically determined growth variation in bivalve larvae (Crassostrea gigas). J Exp Biol 213:749–758
Morgan IJ, McCarthy ID, Metcalfe NB (2000) Life-history strategies and protein metabolism in overwintering juvenile Atlantic salmon: growth is enhanced in early migrants through lower protein turnover. J Fish Biol 56:637–647
Myrand B, Tremblay R, Sevigny JM (2009) Decreases in multi-locus heterozygosity in suspension-cultured mussels (Mytilus edulis) through loss of the more heterozygous individuals. Aquac 295:188–194
Newkirk GF (1980) Review of the genetics and the potential for selective breeding of commercially important bivalves. Aquaculture 19:209–228
Pace DA, Marsh AG, Leong PK, Green AJ, Hedgecock D, Manahan DT (2006) Physiological bases of genetically determined variation in growth of marine invertebrate larvae: a study of growth heterosis in the bivalve Crassostrea gigas. J Exp Mar Biol Ecol 335:188–209
Parry GD (1983) The influence of the costs of growth on ectotherm metabolism. J Theor Biol 101:453–477
Pernet F, Tremblay R, Redjah I, Sévigny JM, Gionet C (2008) Physiological and biochemical traits correlate with differences in growth rate and temperature adaptation among groups of the eastern oyster Crassostrea virginica. J Exp Biol 211:969–977
Riisgård HU (1988) Efficiency of particle retention and filtration rate in 6 species of Northeast American bivalves. Mar Ecol Prog Ser 45:217–223
Riisgård HU (1998) No foundation of a “3/4 power scaling law” for respiration in biology. Ecol Lett 1:71–73
Riisgård HU, Egede PP, Saavedra IB (2011) Feeding behaviour of the mussel, Mytilus edulis: new observations, with mini-review of current knowledge. J Mar Biol 2011:1–13. doi:10.1155/2011/312459
Ropert M, Goulletquer P (2000) Comparative physiological energetics of two suspension feeders: polychaete annelid Lanice conchilega (Pallas 1766) and Pacific cupped oyster Crassostrea gigas (Thunberg 1795). Aquaculture 181:171–189
Sheridan AK (1997) Genetic improvement of oyster production—a critique. Aquaculture 153:165–179
Tamayo D, Ibarrola I, Urrutia MB, Navarro E (2011) The physiological basis for inter-individual growth variability in the spat of clams (Ruditapes philippinarum). Aquaculture 321:113–120
Tamayo D, Ibarrola I, Navarro E (2013) Thermal dependence of clearance and metabolic rates in slow- and fast-growing spats of manila clam Ruditapes philippinarum. J Comp Physiol B 183:893–904
Tanguy A, Bierne N, Saavedra C et al (2008) Increasing genomic information in bivalves through new EST collections in four species: development of new genetic markers for environmental studies and genome evolution. Gene 408:27–36
Teixeira de Sousa J, Matias D, Joaquim S, Ben-Hamadou R, Leitão A (2011) Growth variation in bivalves: new insights into growth, physiology and somatic aneuploidy in the carpet shell Ruditapes decussatus. J Exp Mar Biol Ecol 406:46–53
Thiriot-Quièvreux C, Pogson GH, Zouros E (1992) Genetics of growth rate variation in bivalves: aneuploidy and heterozygosity effects in a Crassostrea gigas family. Genome 35:39–45
Toro JE, Vergara AM (1998) Growth and heterozygosity in a 12-month-old cohort of Ostrea chilensis obtained by mass spawning in laboratory. Mar Ecol 19:311–323
Toro JE, Vergara AM, Gallegillos R (1996) Multiple-locus heterozygosity and growth at two different stages in the life cycle of the Chilean oyster Ostrea chilensis. Mar Ecol Prog Ser 134:151–158
Vladimirova IG, Kleimenov SY, Radzinskaya LI (2003) The relation of energy metabolism and body weight in bivalves. Biol Bull 30:392–399
Whyte J (1987) Biochemical composition and energy content of six species of phytoplankton used in mariculture of bivalves. Aquaculture 60:231–241
Zar JH (1984) Bioestatistical analysis, 3rd edn. Prentice-Hall, Englewood Cliffs
Zhang G, Fang X, Guo X et al (2012) The oyster genome reveals stress adaptation and complexity of shell formation. Nature 490(7418):49–54
Zhao X, Yu H, Kong L, Li Q (2012) Transcriptomic responses to salinity stress in the Pacific oyster Crassostrea gigas. PLoS One 7(9):e46244. doi:10.1371/journal.pone.0046244
Zouros E, Thiriot-Quièvreux C, Kotulas G (1996) The negative correlation between somatic aneuploidy and growth in the oyster Crassostrea gigas and implications for the effects of induced polyploidization. Genet Res 68:109–116
Acknowledgments
This study was funded through the project AGL2009-09981 of the Spanish Ministry of Science and Innovation. D.T. was funded by a FPI grant from the Basque Government. Thanks are given to Juan Cigarría (Tinamenor S.L.) for providing selected specimens of oysters. Technical and human support provided by SGIker (UPV/EHU), MICINN, GV/EJ, ERDF and ESF is gratefully acknowledged. Comments and suggestions of two anonymous reviewers have greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Grassle.
Rights and permissions
About this article
Cite this article
Tamayo, D., Ibarrola, I., Urrutxurtu, I. et al. Physiological basis of extreme growth rate differences in the spat of oyster (Crassostrea gigas). Mar Biol 161, 1627–1637 (2014). https://doi.org/10.1007/s00227-014-2447-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2447-1