Abstract
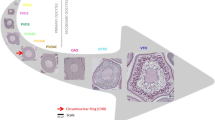
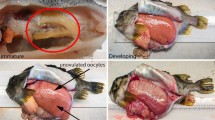
The gonadosomatic index (GSI) is widely used as a simple measure of reproductive capacity, but its validity has often been questioned. This study showed the inter-spawning variation in the predicted GSI of Japanese anchovy using the gravimetric oocyte packing density method. Time course sampling showed that final oocyte maturation and subsequent ovulation occurred from afternoon to evening on the day of spawning. Oocyte in vitro assay, however, suggested that the timing of the spawning could be predetermined by midnight the day before spawning. Models predicted that the GSI of a female just after spawning gradually increases until the completion of germinal vesicle breakdown, after which it dramatically rises by 24–53 % per hour within a few hours of the onset of ovulation. This indicates that the predicted GSI of a female increases by 3.5 times within about 19 h before ovulation, even if the relative batch fecundity remains constant.







Similar content being viewed by others
References
Cubillos LA, Claramunt G (2009) Length-structured analysis of the reproductive season of anchovy and common sardine off central southern Chile. Mar Biol 156:1673–1680
DeVlaming V, Grossman G, Chapman F (1982) On the use of the gonadosomatic index. Comp Biochem Physiol 73:31–39
Erickson DL, Hightower JE, Grossman GD (1985) The relative gonadal index: an alternative index for quantification of reproductive condition. Comp Biochem Physiol 81:117–120
Funamoto T, Aoki I (2002) Reproductive ecology of Japanese anchovy off the Pacific coast of eastern Honshu, Japan. J Fish Biol 61:154–169
Gunderson DR (1997) Trade-off between reproductive effort and adult survival in oviparous and viviparous fishes. Can J Fish Aquat Sci 54:990–998
Hunter JR, Macewicz BJ. (1985) Measurement of spawning frequency in multiple spawning fishes. In: Lasker R (ed) An egg production method for estimating spawning biomass of pelagic fish: application to the Northern Anchovy, Engraulis mordax. NOAA Technical Report NMFS, pp. 79–93
Imai C, Tanaka S (1997) Effect of sea water temperature on variability of batch fecundity in Japanese anchovy from coastal waters around Miura Peninsula, central Japan. Fish Sci 63:489–495
Imai C, Kajitori K, Tajima Y, Nakamura M, Uchiyama M, Yamada H (1998) Biomass estimation of Japanese anchovy stock in the Honshu-Pacific waters by the egg production method using sea surface temperature information. Bull Jpn Soc Fish Oceanogr 62:356–368
Jons GD, Miranda LE (1997) Ovarian weight as an index of fecundity, maturity, and spawning periodicity. J Fish Biol 50:150–156
Kawaguchi K, Yamashita Y, Hayashi A (1990) Some aspects of spawning of the reared Japanese anchovy (Engraulis japonicus H.) in relation to the photoperiod, water temperature and starvation. Bull Jpn Soc Fish Oceanogr 54:364–372
Kitano H, Irie S, Ohta K, Hirai T, Yamaguchi A, Matsuyama M (2011) Molecular cloning of two gonadotropin receptors and their distinct mRNA expression profiles in daily oogenesis of the wrasse Pseudolabrus sieboldi. Gen Comp Endocrinol 172:268–276
Kjesbu OS, Solemdal P, Bratland P, Fonn M (1996) Variation in annual egg production in individual captive Atlantic cod (Gadus morhua). Can J Fish Aquat Sci 53:610–620
Kurita Y, Kjesbu OS (2009) Fecundity estimation by oocyte packing density formulae in determinate and indeterminate spawners: theoretical consideration and applications. J Sea Res 61:188–196
Lambert Y, Yaragina NA, Kraus G, Marteinsdottir G, Wright PJ (2003) Using environmental and biological indices as proxies for egg and larval production of marine fish. J Northw Atl Fish Sci 33:115–159
Motulsky H, Christopoulos A (2003) Fitting models to biological data using linear and nonlinear regression. GrahPad Software Inc, San Diego
Nagahama Y, Yamashita M (2008) Regulation of oocyte maturation in fish. Dev Growth Differ 50:S195–S219
Schismenou E, Somarakis S, Thorsen A, Kjesbu OS (2012) Dynamics of de novo vitellogenesis in fish with indeterminate fecundity: application of oocyte packing density theory to European anchovy, Engraulis encrasicolus. Mar Biol 159:757–768
Shiraishi T, Ketkar SD, Kitano H, Nyuji M, Yamaguchi A, Matsuyama M (2008) Time course of final oocyte maturation and ovulation in chub mackerel Scomber japonicus induced by hCG and GnRHa. Fish Sci 74:764–769
Somarakis S, Ganias K, Tserpes G, Koutsikopoulos C (2004) Ovarian allometry and the use of the gonadosomatic index: a case study in the Mediterranean sardine, Sardina pilchardus. Mar Biol 146:181–189
Stratoudakis Y, Bernal M, Ganias K, Uriarte A (2005) The daily egg production method: recent advances, current application and future challenges. Fish Fish 7:35–57
Takasuka A, Oozeki Y, Kubota H, Tsuruta Y, Funamoto T (2005) Temperature impacts on reproductive parameters of Japanese anchovy: comparison between inshore and offshore waters. Fish Res 76:475–482
Thorsen A, Kjesbu OS (2001) A rapid method for estimation of oocyte size and potential fecundity in Atlantic cod using a computer-aided particle analysis system. J Sea Res 46:295–308
Tsuruta Y (1992) Reproduction in the Japanese anchovy (Engraulis japonica) as related to population fluctuation. Bull Nat Res Inst Fish Eng 13:129–168
Tsuruta Y, Takahashi A (1997) Reproductive ecology of the Japanese anchovy (Engraulis japonicus H.) in the Kuroshio extension and the mixed water region. Bull Hokkaido Nat Fish Res Inst 61:9–15
Weber GM, Sullivan CV (2001) In vitro hormone induction of final oocyte maturation in striped bass (Morone saxatilis) follicles is inhibited by blockers of phosphatidylinositol 3-kinase activity. Comp Biochem Physiol 129B:467–473
West G (1990) Methods of assessing ovarian development in fishes: a review. Aust J Mar Freshw Res 41:199–222
Zenitani H, Kono N, Tsukamoto Y (2005) Effect of sea water temperature and condition factor on variability of spawning interval and relative batch fecundity of Japanese anchovy in the Seto Inland Sea in summer and autumn. Nippon Suisan Gakkaishi 71:821–823
Acknowledgments
We are grateful to the staff and students of the Fishery Research Laboratory and Laboratory of Marine Biology at Kyushu University for their support in rearing and measuring the specimens. We also thank three anonymous reviewers for valuable comments. This research was funded by a subproject on studies on the prediction and application of fish species alternation (SUPRFISH), financed by the Agriculture, Forestry and Fisheries Research Council of Japan, as part of the Population Outbreak of Marine Life (POMAL) Project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Harrod.
Rights and permissions
About this article
Cite this article
Yoneda, M., Kitano, H., Selvaraj, S. et al. Dynamics of gonadosomatic index of fish with indeterminate fecundity between subsequent egg batches: application to Japanese anchovy Engraulis japonicus under captive conditions. Mar Biol 160, 2733–2741 (2013). https://doi.org/10.1007/s00227-013-2266-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-013-2266-9