Abstract
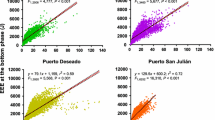
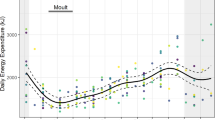
Energy management during the breeding season is crucial for central place foragers since parents need to feed themselves and their offspring while being spatially and temporally constrained. In this work, we used overall dynamic body acceleration as a measure of activity and also to allude to the foraging energy expenditure of breeding Imperial cormorants Phalacrocorax atriceps. We also analyzed how changes in the time or energy allocated to different activities affected the foraging trip energy expenditure and estimated the daily food requirements of the species. Birds spent 42 % of the total energy flying to and from the feeding areas and 16 % floating at sea. The level of activity underwater was almost 1.5 times higher for females than for males. The most expensive diving phase in terms of rate of energy expenditure was descending though the water column. The total foraging trip energy expenditure was particularly sensitive to variation in the amount of time spent flying. During the breeding season, adult cormorants breeding along the Patagonian coast would consume approximately 10,000 tons of food.





Similar content being viewed by others
References
Bishop CM, Butler PJ (1995) Physiological modelling of oxygen consumption in birds during flight. J Exp Biol 198:2153–2163
Brooke ML (2004) The food consumption of the world’s seabirds. P Roy Soc Lond B Bio 271:246–248
Brown JH, Gilloly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology 85:1771–1789
Bulgarella M, Cella Pizarro L, Quintana F, Sapoznikow A, Gosztonyi AE, Kuba L (2008) Diet of Imperial Cormorants (Phalacrocorax atriceps) and rock shags (P. magellanicus) breeding sympatrically in Patagonia, Argentina. Ornitol Neotr 19:553–563
Casaux R, Favero M, Silva P, Baroni A (2001) Sex differences in diving depths and diet of Antarctic shags at the South Shetland Islands. J Field Ornithol 72:22–29
Cook TR, Bailleul F, Lescroël A, Tremblay Y, Bost CA (2008) Crossing the frontier: vertical transit rates of deep diving cormorants reveal depth zone of neutral buoyancy. Mar Biol 154:383–391
Cook TR, Kato A, Tanaka Y, Ropert-Coudert Y, Bost CA (2010) Buoyancy under control: underwater locomotor performance in a deep diving seabird suggest respiratory strategies for reducing foraging effort. PLoS ONE 5:e9839. doi:10.1371/journal.pone.0009839
Culik BM, Wilson RP (1994) Underwater swimming at low energetic cost by Pygoscelid penguins. J Exp Biol 197:65–78
Cury PM, Boyd IL, Bonhommeau S, Anker-Nilssen T, Crawford RJM, Furness RW, Mills JA, Murphy EJ, Österblom H, Paleczny M, Piatt JF, Roux JP, Shannon L, Sydeman WJ (2012) Global seabird response to forage fish depletion—one third for the bird. Science 334:1703–1706
Enstipp MR, Daunt F, Wanless S, Humphreys EM, Hamer KC, Benvenuti S, Grémillet D (2006) Foraging energetics of North Sea birds confronted with fluctuation prey availability. In: Boyd IL, Wanless S, Camphuysen CJ (eds) Top predators in marine ecosystems, their role in monitoring and management. Cambridge University Press, Cambridge, pp 191–210
Favero M, Casaux R, Silva P, Barrera-Oro E, Coria N (1998) The diet of the Antarctic shag during summer at Nelson Island, Antarctica. Condor 100:112–118
Forbes SL, Jajam M, Kaiser GW (2000) Habitat constraints and spatial bias in seabird colony distributions. Ecography 23:575–578
Fossette S, Schofield G, Lilley MKS, Gleiss A, Hays GC (2012) Acceleration data reveal the energy management strategy of a marine ectotherm during reproduction. Funct Ecol 26:324–333
Frere E, Quintana F, Gandini P (2005) Cormoranes de la costa Patagónica: Estado poblacional, ecología y conservación. Hornero 20:35–52
Gleiss AC, Dale JJ, Holland KN, Wilson RP (2010) Accelerating estimates of activity-specific metabolic rate in fishes: testing the applicability of acceleration data-loggers. J Exp Mar Biol Ecol 385:85–91
Gleiss AC, Wilson RP, Shepard ELC (2011) Making overall dynamic body acceleration work; on the theory of acceleration as a proxy for energy expenditure. Methods Ecol Evol 2(1):23–33
Gómez Laich A, Wilson RP, Quintana F, Shepard ELC (2008) Identification of Imperial cormorant Phalacrocorax atriceps behaviour using accelerometers. Endang Spec Res 10:29–37
Gómez Laich A, Wilson RP, Gleiss A, Shepard ELC, Quintana F (2011) Use of overall dynamic body acceleration for estimating energy expenditure in cormorants. Does locomotion in different media affect relationships? J Exp Mar Biol Ecol 399:151–155
Gómez Laich A, Quintana F, Shepard ELC, Wilson RP (2012) Intersexual differences in the diving behaviour of Imperial Cormorants. J Ornith 153:139–147
Gonzalez Miri L, Malacalza V (1999) Perfil nutricional de las principales especies en la dieta del Cormorán Real (Phalacrocorax albiventer) en Punta León (Chubut, Argentina). Ornitol Neotr 10:55–59
Gosztonyi AE, Kuba L (1998) Fishes in the diet of the Imperial cormorant Phalacrocorax atriceps at Punta Lobería Chubut, Argentina. Mar Ornithol 26:59–61
Green J, Halsey LG, Wilson RP, Frappell PB (2009) Estimating energy expenditure of animals using the accelerometry technique: activity, inactivity and comparison with the heart-rate technique. J Exp Biol 212:471–482
Grémillet D, Wright G, Lauder A, Carss DN, Wanless S (2003) Modelling the daily food requirements of wintering great cormorants: a bioenergetics tool for wildlife management. J App Ecol 40:266–277
Halsey LG, Shepard ELC, Hulston CJ, Venables MC, White CR, Jeukendrup AE, Wilson RP (2008a) Acceleration versus heart rate for estimating energy expenditure and speed during locomotion in animals: tests with an easy model species, Homo sapiens. Zoology 111:231–241
Halsey LG, Shepard ELC, Quintana F, Gómez Laich A, Green JA, Wilson RP (2008b) The relationship between oxygen consumption and body acceleration in a range of species. Comp Biochem Phys A 152:197–202
Halsey LG, Green JA, Wilson RP, Frappell PB (2009) Accelerometry to estimate energy expenditure during activity: best practice with data loggers. Physiol Biochem Zool 82:396–404
Halsey LG, Shepard ELC, Wilson RP (2011) Assessing the development and application of the accelerometry technique for estimating energy expenditure. Comp Biochem Physiol Part A 158:305–314
Harris S, Quintana F, Raya Rey A (2012) Prey Search behaviour of the Imperial Cormorant (Phalacrocorax atriceps) during the breeding season at Punta León, Argentina. Waterbirds 35:312–323
Hedenstrom A, Alerstam T (1995) Optimal flight speed of birds. Philos Trans R Soc Lond B Biol Sci 348:471–487
Kacelnik A (1984) Central place foraging in starlings (Sturnus-vulgaris).1. Patch residence time. J Anim Ecol 53:283–299
Kato A, Nishiumi I, Naito Y (1996) Sexual differences in the diet of king cormorants at Macquarie Island. Polar Biol 16:75–77
Kato A, Watanuki Y, Shaughnessy P, Le Maho Y, Naito Y (1999) Intersexual differences in the diving behaviour of foraging subantarctic cormorant (Phalacrocorax albiventer) and Japanese cormorant (P. filamentosus). CR Acad Sci 322:557–562
Kato A, Watanuki Y, Nishiumi I, Kuroki M, Shaughnessy P, Naito Y (2000) Variation in foraging and parental behavior of king cormorants. Auk 117:718–730
Kramer DL (1988) The behavioural ecology of air breathing by aquatic animals. Can J Zool 66:89–94
Elliot KH, Le Vaillant, M, Kato A, Speakman JR, Ropert Coudert Y (2013) Accelerometry predicts daily energy expenditure in a bird with high activity levels. Biol Lett. doi:10.1098/rsbl.2012.09191744-957X
Liordos V, Goutner V (2009) Sexual differences in the diet of great cormorants Phalacrocorax carbo sinensis wintering in Greece. Eur J Wildl Res 55:301–308
Malacalza VE, Hall MA (1988) Sexing adult King Cormorants (Phalacrocorax albiventer) by discriminant analysis. Colon Waterbird 11:32–37
Orians GH, Pearson NE (1979) On the theory of central place foraging. In: Horn DJ, Mitchell RD, Stairs GR (eds) Analysis of ecological systems. Ohio State University Press, Columbus, pp 154–177
Orta J (1992) Family Phalacrocoracidae (Cormorants). In: del Hoyo J, Elliott A, Sargatal J (eds) Handbook of the birds of the world, vol 1. Barcelona, Lynx Edicions, pp 326–353
Pennycuick CJ (2008) Modelling the flying bird. Elsevier, Amsterdam
Punta GE, Saravia JRC, Yorio PM (1993) The diet and foraging behaviour of two patagonian cormorants. Mar Ornithol 21:27–36
Punta GE, Yorio P, Herrera G, Saravia JCR (2003) Biología reproductiva de los cormoranes imperial (Phalacrocorax atriceps) y cuello negro (P. magellanicus) en el golfo San Jorge, Chubut, Argentina. Hornero 18:103–111
Quintana F, Wilson R, Dell′Arciprete P, Shepard E, Gómez Laich A (2011) Women from Venus, men from Mars: inter-sex foraging differences in the Imperial cormorant Phalacrocorax atriceps a colonial seabird. Oikos 120:350–358
R Development Core Team I (2008) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Schmidt-Nielsen K (1990) Animal physiology: adaptation and environment. Cambridge University Press, Cambridge
Schreiber EA, Burger J (2002) Biology of marine birds. CRC Press LL, Florida
Shepard ELC, Wilson MP, Quintana F, Gómez Laich A, Liebsch N, Albareda D, Halsey LG, Gleiss A, Morgan DT, Myers AE, Newman C, Macdonald DW (2008) Identification of animal movement patterns using tri-axial accelerometry. Endang Spec Res 10:47–60
Shepard ELC, Wilson RP, Quintana F, Gómez Laich A, Forman DW (2009) Pushed for time or saving fuel: fine-scale energy budgets shed light on currencies in a diving bird. Proc R Soc B 276:3149–3155
Shepard ELC, Wilson RP, Gómez Laich A, Quintana F (2010) Buoyed up and slowed down: sped limits for diving birds in shallow water. Aquat Biol 8:259–267
Stearns SC (1992) The evolution of life history. Oxford University Press, New York
Svagelj W, Quintana F (2007) Sexual size dimorphism and sex determination by morphometric measurements in breeding shags (Phalacrocorax atriceps). Waterbirds 30:97–102
Svagelj W, Quintana F (2011) Breeding performance of the Imperial Shag (Phalacrocorax atriceps) in relation to year, laying date and nest location. Emu 111:162–165
Wanless S, Harris MP, Morris JA (1992) Diving behavior and diet of the blue-eyed shag at South-Georgia. Polar Biol 12:713–719
Watanabe YY, Takahashi A, Sato K, Viviant M, Bost CA (2011) Poor flight performance in deep-diving cormorants. J Exp Biol 214:412–421
Wilson RP, Quintana F (2004) Surface pauses in relation to dive duration in imperial cormorants; how much time for a breather? J Exp Biol 207:1789–1796
Wilson RP, Hustler K, Ryan PG, Noeldeke C, Burger AE (1992) Diving birds in cold water: do Archimedes and Boyle determine energy costs? Am Nat 140:179–200
Wilson RP, Putz K, Charrassin JB, Lage J (1997) Long-term attachment of transmitting and recording devices to penguins and other seabirds. Wildlife Soc B 25:101–106
Wilson RP, Steinfurth A, Ropert-Coudert Y, Kato A, Kurita M (2002) Lip-reading in remote subjects: an attempt to quantify and separate ingestion, breathing and vocalisation in free-living animals using penguins as a model. Mar Biol 140:17–27
Wilson RP, White CR, Quintana F, Halsey LG, Liebsh N, Martin GR, Butler PJ (2006) Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. J Anim Ecol 75:1081–1090
Wilson RP, Shepard ELC, Liebsch N (2008a) Prying into the intimate details of animal lives: use of a daily diary on animals. Endang Spec Res 4:123–137
Wilson RP, Vargas FH, Steinfurth A, Riordan P, Ropert-Coudert Y, Macdonald DW (2008b) What grounds some birds for life? Movement and diving in the sexual dimorphic Galápagos Cormorant. Ecol Monogr 78:633–652
Wilson RP, McMahon CR, Quintana F, Frere E, Scolaro A, Hays GC, Bradshaw CJA (2011a) N-dimensional animal energetic niches clarify behavioral options in a variable marine environment. J Exp Biol 214:646–656
Wilson RP, Quintana F, Hobson VJ (2011b) Construction of energy landscapes can clarify the movement and distribution of foraging animals. P Natl Acad Sci 279:975–980
Yoda K, Naito Y, Sato K, Takahashi A, Nishikawa J, Ropert-Coudert Y, Kurita M, Le Maho Y (2001) A new technique for monitoring the behaviour of free-ranging Adelie penguins. J Exp Biol 204:685–690
Yorio P, Copello S, Kuba L, Gosztonyi A, Quintana F (2010) Diet of Imperial Cormorants, Phalacrocorax atriceps, breeding at Central Patagonia, Argentina. Waterbirds 33:70–78
Acknowledgments
This research was funded by grants from the Wildlife Conservation Society, Consejo Nacional de Investigaciones Científicas y Técnicas de la República Argentina (CONICET) and Agencia de Promoción Científica y Tecnológica to F. Quintana and by a Rolex Award for Enterprise awarded to R.P. Wilson. We would like to thank the Organismo Provincial de Turismo for the permits to work in Punta León and the Centro Nacional Patagónico (CENPAT-CONICET) for institutional and logistical support. A. Gómez-Laich is supported by a Postdoctoral fellowship from the Consejo Nacional de Investigaciones Científicas y Técnicas de la República Argentina (CONICET).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Garthe.
Rights and permissions
About this article
Cite this article
Gómez-Laich, A., Wilson, R.P., Shepard, E.L.C. et al. Energy expenditure and food consumption of foraging Imperial cormorants in Patagonia, Argentina. Mar Biol 160, 1697–1707 (2013). https://doi.org/10.1007/s00227-013-2222-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-013-2222-8