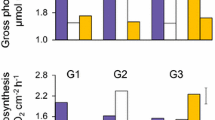
Abstract
Recently, it has been suggested that there are conditions under which some coral species appear to be resistant to the effects of ocean acidification. To test if such resistance can be explained by environmental factors such as light and food availability, the present study investigated the effect of 3 feeding regimes crossed with 2 light levels on the response of the coral Porites rus to 2 levels of pCO2 at 28 °C. After 1, 2, and 3 weeks of incubation under experimental conditions, none of the factors—including pCO2—significantly affected area-normalized calcification and biomass-normalized calcification. Biomass also was unaffected during the first 2 weeks, but after 3 weeks, corals that were fed had more biomass per unit area than starved corals. These results suggest that P. rus is resistant to short-term exposure to high pCO2, regardless of food availability and light intensity. P. rus might therefore represent a model system for exploring the genetic basis of tolerance to OA.

Similar content being viewed by others
References
Adam TC, Schmitt RJ, Holbrook SJ, Brooks AJ, Edmunds PJ, Carpenter RC, Bernardi G (2011) Herbivory, connectivity, and ecosystem resilience: response of a coral reef to a large-scale perturbation. PLoS ONE 6:e23717. doi:10.1371/journal.pone.0023717
Adjeroud M (1997) Factors influencing spatial patterns on coral reefs around Moorea, French Polynesia. Mar Ecol Prog Ser 159:105–119. doi:10.3354/meps159105
Alldredge A, Carlson C of Moorea Coral Reef LTER (2011) MCR LTER: Coral reef: Water column: Nearshore water profiles, CTD, primary production, and chemistry. knb-lter-mcr.10.29 (http://metacat.lternet.edu/knb/metacat/knb-lter-mcr.10.29/lter)
Anthony KRN (1999) Coral suspension feeding on fine particulate matter. J Exp Mar Biol Ecol 232:85–106. doi:10.1016/S0022-0981(98)00099-9
Anthony KRN (2000) Enhanced particle-feeding capacity of corals on turbid reefs (Great Barrier Reef, Australia). Coral Reefs 19:59–67. doi:10.1007/s003380050227
Anthony KRN, Fabricius KE (2000) Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. J Exp Mar Biol Ecol 252:221–253. doi:10.1016/S0022-0981(00)00237-9
Anthony KRN, Connolly SR, Willis BL (2002) Comparative analysis of energy allocation to tissue and skeletal growth in corals. Limnol Oceanogr 47:1417–1429. doi:10.4319/lo.2002.47.5.1417
Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O (2008) Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc Natl Acad Sci 105:17442–17446. doi:10.1073/pnas.0804478105
Atkinson MJ, Cuet P (2008) Contribution to the theme section “Effects of ocean acidification on marine ecosystems” possible effects of ocean acidification on coral reef biogeochemistry: topics for research. Mar Ecol Prog Ser 373:249–256. doi:10.3354/meps07867
Atkinson MJ, Carlson B, Crow GL (1995) Coral growth in high-nutrient, low-pH seawater: a case study of corals cultured at the Waikiki Aquarium, Honolulu, Hawaii. Coral Reefs 14:215–223. doi:10.1007/BF00334344
Caldeira K, Wickett ME (2003) Oceanography: anthropogenic carbon and ocean pH. Nature 425:365. doi:10.1038/425365a
Comeau S, Edmunds PJ, Spindel NB, Carpenter RC (2013a) The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol Oceanogr (in press)
Comeau S, Carpenter RC, Edmunds PJ (2013b) Coral reef calcifiers buffer their response to ocean acidification using both bicarbonate and carbonate. Proc Roy Soc B 280. doi:10.1098/rspb.2012.2374
Crawley A, Kline DI, Dunn S, Anthony K, Dove S (2010) The effect of ocean acidification on symbiont photorespiration and productivity in Acropora formosa. Glob Change Biol 16:851–863. doi:10.1111/j.1365-2486.2009.01943.x
Davies PS (1989) Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar Biol 101:389–395
Dickson AG, Sabine CL, Christian JR (eds) (2007) Guide to best prac- tices for ocean CO2 measurements. PICES Special Publication 3
Edmunds PJ (2011) Zooplanktivory ameliorates the effects of ocean acidification on the reef coral Porites spp. Limnol Oceanogr 56:2402–2410
Edmunds P (2012) Effect of pCO2 on the growth, respiration, and photophysiology of massive Porites spp. in Moorea, French Polynesia. Mar Biol:1–12. doi:10.1007/s00227-012-2001-y
Edmunds PJ, Brown D, Moriarty V (2012) Interactive effects of ocean acidification and temperature on two scleractinian corals from Moorea, French Polynesia. Glob Change Biol 18:2173–2183. doi:10.1111/j.1365-2486.2012.02695.x
Gattuso J-P, Allemand D, Frankignoulle M (1999) Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry. Am Zool 39:160–183. doi:10.1093/icb/39.1.160
Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, Gomez E, Harvell CD et al (2007) Coral reefs under rapid climate change and ocean acidification. Science 318:1737–1742. doi:10.1126/science.1152509
Holcomb M, McCorkle DC, Cohen AL (2010) Long-term effects of nutrient and CO2 enrichment on the temperate coral Astrangia poculata (Ellis and Solander, 1786). J Exp Mar Biol Ecol 386:27–33. doi:10.1016/j.jembe.2010.02.007
Houlbrèque F, Ferrier-Pagès C (2009) Heterotrophy in tropical scleractinian corals. Biol Rev 84:1–17. doi:10.1111/j.1469-185X.2008.00058.x
Houlbreque F, Tambutte E, Richard C, Ferrier-Pages C (2004) Importance of a micro-diet for scleractinian corals. Mar Ecol Prog Ser 282:151–160
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R et al (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933. doi:10.1126/science.1085046
Kleypas J, Yates K (2009) Coral reefs and ocean acidification. Oceanography 22:108–117. doi:10.5670/oceanog.2009.101
Langdon C, Atkinson MJ (2005) Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/irradiance and nutrient enrichment. J Geophys Res 110:C09S07. doi:10.1029/2004JC002576
Lavigne H, Gattuso J-P (2011) seacarb: seawater carbonate chemistry with R. R package version 2.4.1. http://CRAN.Rproject.org/package=seacarb
Marsh JA (1970) Primary productivity of reef-building calcareous red algae. Ecology 51:255–263. doi:10.2307/1933661
Marubini F, Barnett H, Langdon C, Atkinson MJ (2001) Dependence of calcification on light and carbonate ion concentration for the hermatypic coral Porites compressa. Mar Ecol Prog Ser 220:153–162
McCulloch M, Falter J, Trotter J, Montagna P (2012) Coral resilience to ocean acidification and global warming through pH up-regulation. Nat Clim Change 2:623–627
Mills MM, Lipschultz F, Sebens KP (2004) Particulate matter ingestion and associated nitrogen uptake by four species of scleractinian corals. Coral Reefs 23:311–323. doi:10.1007/s00338-004-0380-3
Moss RH, Edmonds JA, Hibbard KA, Manning MR, Rose SK, van Vuuren DP, Carter TR et al (2010) The next generation of scenarios for climate change research and assessment. Nature 463:747–756. doi:10.1038/nature08823
Muscatine L (1990) The role of symbiotic algae in carbon and energy flux in reef corals. In: Dubinsky Z (ed) Ecosystems of the world. Elsevier, Amsterdam, pp 75–87
Orr JC (2011) Recent and future changes in ocean carbonate chemistry. In: Gattuso J-P, Hansson L (eds) Ocean acidification. Oxford University Press, Oxford, pp 41–66
Pandolfi JM, Connolly SR, Marshall DJ, Cohen AL (2011) Projecting coral reef futures under global warming and ocean acidification. Science 333:418–422. doi:10.1126/science.1204794
Quinn GP, Keough MJ (eds) (2002) Experimental design and data analysis for biologists. Cambridge University Press, Melbourne
Renegar DA, Riegl BM (2005) Effect of nutrient enrichment and elevated CO2 partial pressure on growth rate of Atlantic scleractinian coral Acropora cervicornis. Mar Ecol Prog Ser 293:69–76. doi:10.3354/meps293069
Reynaud S, Leclercq N, Romaine-Lioud S, Ferrier-Pagés C, Jaubert J, Gattuso J-P (2003) Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification in a scleractinian coral. Glob Change Biol 9:1660–1668
Ries JB (2011) A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification. Geochim Cosmochim Acta 75:4053–4064. doi:10.1016/j.gca.2011.04.025
Schneider K, Erez J (2006) The effect of carbonate chemistry on calcification and photosynthesis in the hermatypic coral Acropora eurystoma. Limnol Oceanogr 51:1284–1293
Stambler N (2011) Zooxanthellae: the yellow symbionts inside animals. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Netherland, pp 87–106
Acknowledgments
We thank D. Brown and B. Lenz for field and laboratory assistance, NSF for financial support (OCE 10-41270), and the Moorea Coral Reef Long Term Ecological Research site (OCE 04-17413 and 10-26852) as well as the Gordon and Betty Moore Foundation for logistic support. This is contribution number 190 of the CSUN Marine Biology Program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. G. Chapman.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Comeau, S., Carpenter, R.C. & Edmunds, P.J. Effects of feeding and light intensity on the response of the coral Porites rus to ocean acidification. Mar Biol 160, 1127–1134 (2013). https://doi.org/10.1007/s00227-012-2165-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2165-5