Abstract
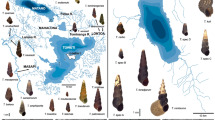
Twenty-two Mya arenaria samples spanning seven marine ecoregions mostly situated in the Cold Temperate Northwest Atlantic (CTNA) biogeographic province were collected between 2001 and 2010 and genotyped at seven highly polymorphic microsatellite loci to test for population differentiation. Results showed strong regional differentiation with six genetic clusters: (1) Northern Gulf of St. Lawrence (GSL), (2) Magdalen Archipelago, (3) Southern GSL, (4) Lower Atlantic Canada, (5) US Coasts and (6) Northern Europe. Population structure was supported no matter the statistical approach and generally does not reflect the geographical limits of marine ecoregions. A latitudinal cline in allelic richness provides evidence for a northward post-glacial expansion range for this species. While geographical distance explains the genetic variation detected in southern CTNA, increased heterogeneity observed in northern CTNA can be explained by isolation by distance, marine landscaping and presumable selective processes acting at the Mar5 locus. Exclusion of Mar5 from analyses resulted in the detection of three genetic clusters instead of six.







Similar content being viewed by others
References
Archambault P, Snelgrove PVR, Fisher JAD et al (2010) From sea to sea: Canada’s three oceans of biodiversity. PLoS ONE 5:e12182
Baker P, Austin JD, Bowen BW, Baker SM (2008) Range-wide population structure and history of the northern quahog (Mercenaria mercenaria) inferred from mitochondrial DNA sequence data. ICES J Mar Sci 65:155–163
Beaumont MA, Nichols RA (1996) Evaluating loci for use in the genetic analysis of population structure. Proc R Soc B Biol Sci 263:1619–1626
Belkhir K, Borsa P, Chikhi L, Raufaste N, Bonhomme F (1996) GENETIX 4.05, logiciel sous Windows™ pour la génétique des populations. Université de Montpellier II, Montpellier
Bernatchez P, Dubois JMM, Dionne JC (1999) Holocene shell beds of Baie-Comeau along the North shore of the St. Lawrence estuary (Quebec). Can J Earth Sci 36:519–531
British Oceanographic Data Centre (2003) Centenary edition of the GEBCO Digital Atlas. Published on behalf of the International Hydrographic Organization (IHO) and the International Oceanographic Commission (IOC) of UNESCO, Liverpool, UK
Camilli L, Castelli A, Lardicci C, Maltagliati F (2001) Evidence for high levels of genetic divergence between populations of the bivalve Mytilaster minimus from a brackish environment and two adjacent marine sites. J Molluscan Stud 67:506–510
Caporale DA, Beal BF, Roxby R, VanBeneden RJ (1997) Population structure of Mya arenaria along the New England coastline. Mol Mar Biol Biotech 6:33–39
Cassista MC, Hart MW (2007) Spatial and temporal genetic homogeneity in the Arctic surfclam (Mactromeris polynyma). Mar Biol 152:569–579
Chabot D, Rondeau A, Sainte-Marie B, Savard L, Surette T, Archambault P (2007) Distribution of benthic invertebrates in the Estuary and Gulf of St. Lawrence. Can Sci Advis Secr 2007/018
Chatterji S, Pachter L (2006) Reference based annotation with GeneMapper. Genome Biol 7:R29
Cockerham CC, Weir BS (1993) Estimation of gene flow from F-statistics. Evolution 47:855–863
Congleton WR, Pearce BR, Parker MR, Causey RC (2003) Mariculture siting—tidal currents and growth of Myaarenaria. J Shellfish Res 22:75–83
Crawford NG (2010) SMOGD: software for the measurement of genetic diversity. Mol Ecol Resour 10:556–557
Dahlgren TG, Weinberg JR, Halanych KM (2000) Phylogeography of the ocean quahog (Arctica islandica): influences of paleoclimate on genetic diversity and species range. Mar Biol 137:487–495
Dai A, Trenberth KE (2002) Estimates of freshwater discharge from continents: latitudinal and seasonal variations. J Hydrometeorol 3:660–687
Department of Fisheries and Oceans Canada (2009) Development of a framework and principles for the biogeographic classification of Canadian Marine Areas. Can Sci Advis Secr 2009/056
Domingues CP, Creer S, Taylor MI, Queiroga H, Carvalho GR (2010) Genetic structure of Carcinus maenas within its native range: larval dispersal and oceanographic variability. Mar Ecol Prog Ser 410:111–123
Drouin C-A, Bourget E, Tremblay R (2002) Larval transport processes of barnacle larvae in the vicinity of the interface between two genetically different populations of Semibalanus balanoides. Mar Ecol Prog Ser 229:165–172
Dupont L, Ellien C, Viard F (2007) Limits to gene flow in the slipper limpet Crepidula forniacata as revealed by microsatellite data and a larval dispersal model. Mar Ecol Prog Ser 349:125–138
Dyke AS, Prest VK (1987) Late Wisconsinan and Holocene history of the Laurentide Ice Sheet. Géogr Phys Quat 41:237–263
Elderkin CL, Klerks PL (2001) Shifts in allele and genotype frequencies in zebra mussels, Dreissena polymorpha, along the latitudinal gradient formed by the Mississippi River. J N Am Benthol Soc 20:595–605
Engle VD, Summers JK (1999) Latitudinal gradients in benthic community composition in Western Atlantic estuaries. J Biogeogr 26:1007–1023
Evanno G, Regnaut S, Goudet J (2005) Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol 14:2611–2620
Falush D, Stephens M, Pritchard JK (2003) Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics 164:1567–1587
Fogarty MJ, Botsford LW (2007) Population connectivity and spatial management of marine fisheries. Oceanography 20:112–123
Gan J, Ingram RG, Greatbatch RJ, van der Baaren T (2004) Variability of circulation induced by the separation of Gaspe Current in Baie des Chaleurs (Canada): observational studies. Estuar Coast Shelf Sci 61:393–402
Glaubitz JC (2004) CONVERT: a user-friendly program to reformat diploid genotypic data for commonly used population genetic software packages. Mol Ecol Notes 4:309–310
Goudet J (2001) FSTAT, a program to estimate and test gene diversities and fixation indices Version 2.9.3. http://www2.unil.ch/popgen/softwares/fstat.htm
Goudet J, Raymond M, de-Meeus T, Rousset F (1996) Testing differentiation in diploid populations. Genetics 144:1933–1940
Hedrick PW (2005) A standardized genetic differentiation measure. Evolution 59:1633–1638
Hubisz MJ, Falush D, Stephens M, Pritchard JK (2009) Inferring weak population structure with the assistance of sample group information. Mol Ecol Resour 9:1322–1332
Jensen JL, Bohonak AJ, Kelley ST (2005) Isolation by distance, web service. BMC Genet 6:13–18
Jost L (2008) G(ST) and its relatives do not measure differentiation. Mol Ecol 17:4015–4026
Kenchington EL, Patwary MU, Zouros E, Bird CJ (2006) Genetic differentiation in relation to marine landscape in a broadcast-spawning bivalve mollusc (Placopecten magellanicus). Mol Ecol 15:1781–1796
Kenchington EL, Harding GC, Jones MW, Prodöhl PA (2009) Pleistocene glaciation events shape genetic structure across the range of the American lobster, Homarus americanus. Mol Ecol 18:1654–1667
Kimura M, Crow JF (1964) The number of alleles that can be maintained in a finite population. Genetics 49:725–738
Kimura M, Weiss GH (1964) Stepping stone model of population structure and the decrease of genetic correlation with distance. Genetics 49:561–576
Krakau M, Jacobsen S, Jensen KT, Reise K (2012) The cockle Cerastoderma edule at Northeast Atlantic shores: genetic signatures of glacial refugia. Mar Biol 159:221–230
Lachance AA, Myrand B, Tremblay R, Koutitonsky V, Carrington E (2008) Biotic and abiotic factors influencing attachment strength of blue mussels Mytilus edulis in suspended culture. Aquatic Biol 2:119–129
Larsson LC, Laikre L, Palm S, Andre C, Carvalho GR, Ryman N (2007) Concordance of allozyme and microsatellite differentiation in a marine fish, but evidence of selection at a microsatellite locus. Mol Ecol 16:1135–1147
Lasota R, Hummel H, Wolowicz M (2004) Genetic diversity of European populations of the invasive soft-shell clam Mya arenaria (Bivalvia). J Mar Biol Assoc UK 84:1051–1056
Lutz RA, Jablonski D (1978) Larval bivalve shell morphometry: a new paleoclimatic tool? Science 202:51–53
MacNeil FS (1965) Evolution and distribution of the genus Mya, and Tertiary migrations of Mollusca. Geol Surv Prof Pap 483-G
Maggs CA, Castilho R, Foltz D et al (2008) Evaluating signatures of glacial refugia for North Atlantic benthic marine taxa. Ecology 89:S108–S122
Manni F, Guerard E, Heyer E (2004) Geographic patterns of (genetic, morphologic, linguistic) variation: how barriers can be detected by using Monmonier’s algorithm. Hum Biol 76:173–190
Matschiner M, Salzburger W (2009) TANDEM: integrating automated allele binning into genetics and genomics workflows. Bioinformatics 25:1982–1983
Maximovich NV, Guerassimova AV (2003) Life history characteristics of the clam Mya arenaria in the White Sea. Helgoland Mar Res 57:91–99
Michinina SR, Rebordinos L (1997) Genetic differentiation in marine and estuarine natural populations of Crassostrea angulata. Mar Ecol Prog Ser 154:167–174
Mileikovsky SA (1971) Types of larval development in marine bottom invertebrates, their distribution and ecological significance: a re-evaluation. Mar Biol 10:193–213
Molecular Ecology Resources Primer Development Consortium (2011) Permanent genetic resources added to molecular ecology resources database 1(June), pp. 2011–31, July 2011. Mol Ecol Resour 11:1124–1126
Monmonier M (1973) Maximum-difference barriers: an alternative numerical regionalization method. Geogr Anal 3:245–261
Nei M (1978) Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 89:583–590
Panova M, Blakeslee AMH, Miller AW, Mäkinen T, Ruiz GM, Johannesson K, André C (2011) Glacial history of the North Atlantic marine snail, Littorina saxatilis, inferred from distribution of mitochondrial DNA lineages. PLoS ONE 6:e17511
Paul A, Schäfer-Neth C (2003) Modelling the water masses of the Atlantic Ocean at the last glacial maximum. Paleoceanography 18:1058–1084
Pietrafesa LJ, Morrison JM, McCann MP, Churchill J, Bohm E, Houghton RW (1994) Water mass linkages between the Middle and South Atlantic bights. Deep Sea Res II 41:365–389
Pineira J, Quesada H, Rolan-Alvarez E, Caballero A (2008) Genetic discontinuity associated with an environmentally induced barrier to gene exchange in the marine snail Littorina saxatilis. Mar Ecol Prog Ser 357:175–184
Ponurovskii SK, Kolotukhina NK (2000) Larval settlement of the bivalves Mya arenaria and M. uzenensis from scallop collectors in Vostok Bay, Sea of Japan. Russian J Mar Biol 26:330–337
Powers SP, Bishop MA, Grabowski JH, Peterson CH (2006) Distribution of the invasive bivalve Mya arenaria L. on intertidal flats of southcentral Alaska. J Sea Res 55:207–216
Pritchard JK, Stephens M, Donnelly P (2000) Inference of population structure using multilocus genotype data. Genetics 155:945–959
Raymond M, Rousset F (1995) GENEPOP (version1.2): population genetics software for exact tests and ecumenicism. J Hered 86:248–249
Redjah I, Olivier F, Tremblay R, Myrand B, Pernet F, Neumeier U, Chevarie L (2010) The importance of turbulent kinetic energy on transport of juvenile clams (Mya arenaria). Aquaculture. 307:20–28
Rousset F (1997) Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 145:1219–1228
Rousset F (2008) GENEPOP’ 007: a complete re-implementation of the GENEPOP software for Windows and Linux. Mol Ecol Resour 8:103–106
Santos S, Cruzeiro C, Olsen JL, van der Veer HW, Luttikhuizen PC (2012) Isolation by distance and low connectivity in the peppery furrow shell Scrobicularia plana (Bivalvia). Mar Ecol Prog Ser 462:111–124
Savenkoff C, Vézina AF, Smith PC, Han G (2001) Summer transports of nutrients in the Gulf of St. Lawrence estimated by inverse modelling. Estuar Coast Shelf Sci 52:565–587
Schmidt PS, Rand DM (2001) Adaptive maintenance of genetic polymorphism in an intertidal barnacle: habitat- and life-stage-specific survivorship of Mpi genotypes. Evolution 55:1336–1344
Schmidt PS, Bertness MD, Rand DM (2000) Environmental heterogeneity and balancing selection in the acorn barnacle Semibalanus balanoides. Proc R Soc B Biol Sci 267:379–384
Schmidt PS, Serrão EA, Pearson GA et al (2008) Ecological genetics in the North Atlantic: environmental gradients and adaptation at specific loci. Ecology 89:S91–S107
Schunter C, Carreras-Carbonell J, Macpherson E et al (2011) Matching genetics with oceanography: directional gene flow in a Mediterranean fish species. Mol Ecol 20:5167–5181
Selkoe KA, Toonen RJ (2006) Microsatellites for ecologists: a practical guide to using and evaluating microsatellite markers. Ecol Lett 9:615–629
Sevigny JM, Valentin A, Talbot A, Menard N (2009) Connectivité entre les populations du fjord du Saguenay et celles du Golfe du Saint-Laurent. Rev Sci Eau 22:315–339
Shanks AL (2009) Pelagic larval duration and dispersal distance revisited. Biol Bull 216:373–385
Shearman RK, Lentz SJ (2010) Long-term sea surface temperature variability along the US East Coast. J Phys Oceanogr 40:1004–1017
Sheng J (2001) Dynamics of a buoyancy-driven coastal jet: the Gaspé Current. J Phys Oceanogr 31:3146–3162
Shiah F-K, Ducklow HW (1994) Temperature regulation of heterotrophic bacterioplankton abundance, production, and specific growth rate in Chesapeake Bay. Limnol Oceanogr 39:1243–1258
Shore JA, Hannah CG, Loder JW (2000) Drift pathways on the western Scotian Shelf and its environs. Can J Fish Aquatic Sci 57:2488–2505
Skold M, Wing SR, Mladenov PV (2003) Genetic subdivision of a sea star with high dispersal capability in relation to physical barriers in a fjordic seascape. Mar Ecol Prog Ser 250:163–174
Slatkin M (1985) Rare alleles as indicators of gene flow. Evolution 39:53–65
Sokal RR, Rohlf FJ (1981) Biometry, 2nd edn. W.H Freeman and Company, New York, NY
Spalding MD, Fox HE, Halpern BS et al (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 57:573–583
St-Onge P, Miron G (2007) Effects of current speed, shell length and type of sediment on the erosion and transport of juvenile softshell clams (Mya arenaria). J Exp Mar Biol Ecol 349:12–26
St-Onge P, Parent E, Sevigny JM, Tremblay R, Rioux-Pare R (2011) Isolation and characterization of eight novel microsatellite markers for the softshell clam (Mya arenaria). Mol Ecol Resour Primer Database mer-11-0122
Strasser CA, Barber PH (2009) Limited genetic variation and structure in softshell clams (Mya arenaria) across their native and introduced range. Conserv Genet 10:803–814
Suneetha KB, Naevdal G (2001) Genetic and morphological stock structure of the pearlside, Maurolicus muelleri (Pisces, Sternoptychidae), among Norwegian fjords and offshore area. Sarsia 86:191–201
Thorson G (1950) Reproductive and larval ecology of marine bottom invertebrates. Biol Rev 25:1–45
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Vézina AF, Gratton Y, Vinet P (1995) Mesoscale physical—biological variability during a summer phytoplankton bloom in the lower St. Lawrence Estuary. Estuar Coast Shelf Sci 41:393–411
Vuilleumier S, Goudet J, Perrin N (2010) Evolution in heterogeneous populations: from migration models to fixation probabilities. Theor Popul Biol 78:250–258
Waples RS (1989) Temporal variation in allele frequencies—testing the right hypothesis. Evolution 43:1236–1251
Wares JP (2002) Community genetics in the Northwestern Atlantic intertidal. Mol Ecol 11:1131–1144
Wares JP, Cunningham CW (2001) Phylogeography and historical ecology of the North American intertidal. Evolution 55:2455–2469
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Westgaard JI, Fevolden SE (2007) Atlantic cod (Gadus morhua L.) in inner and outer coastal zones of northern Norway display divergent genetic signature at non-neutral loci. Fish Res 85:306–315
Wright S (1931) Evolution in Mendelian populations. Genetics 16:97–159
Wright S (1943) Isolation by distance. Genetics 28:114–138
Yasuda N, Nagai S, Hamaguchi M, Okaji K, Gerard K, Nadaoka K (2009) Gene flow of Acanthaster planci (L.) in relation to ocean currents revealed by microsatellite analysis. Mol Ecol 18:1574–1590
Young AMC, Torres C, Mack JE, Cunningham CW (2002) Morphological and genetic evidence for vicariance and refugium in Atlantic and Gulf of Mexico populations of the hermit crab Pagurus longicarpus. Mar Biol 140:1059–1066
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Upper Saddle, NJ
Zuccarello GC, West JA (2003) Multiple cryptic species: molecular diversity and reproductive isolation in the Bostrychia radicans/B. moritziana complex (Rhodomelaceae, Rhodophyta) with focus on North American isolates. J Phycol 39:948–959
Acknowledgments
Authors wish to thank the following people for their much appreciated help in the field and in the laboratory: Éric Parent, Éric Tremblay, Léophane Leblanc, Firmin Leblanc, Gilles Miron, Julie Quimper, Alexandra Valentin, Philippe Galipeau, Chantale Daigle, Carole Degrâce, Andre Siah, Sylvie Brulotte, Patrice Pelletier, the Kouchibouguac, Kejimkujik and Cape Breton Highlands National Parks and the Atlantic Veterinary College. Authors would also like to thank all anonymous reviewers who commented on earlier drafts of the manuscript. This study was funded by the Aquaculture Collaborative Research and Development Program (ACRDP), the National Sciences and Engineering Research Council of Canada (NSERC) and the Réseau Aquaculture du Québec (RAQ) grants to P. St-Onge, J.M. Sévigny and R. Tremblay.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Reusch.
Electronic supplementary material
Below is the link to the electronic supplementary material.
227_2012_2157_MOESM9_ESM.docx
Online Resource 9 Assessment of the most probable number of clusters (K) explaining the genetic structure of M. arenaria individuals (N = 604) from 22 samples genotyped with seven microsatellite loci as detected with the ΔK graphical method developed by Evanno et al. (2005). a) Mean (±SD; N = 4) posterior probability of data; b) mean rate (±SD; N = 4) of change of the likelihood distribution; c) mean (±SD; N = 4) absolute values of the second-order rate of change of the likelihood distribution; d) ΔK. Arrows point to the greatest value of ΔK and the most probable number of clusters (K = 6) explaining the genetic structure in the dataset (DOCX 138 kb)
227_2012_2157_MOESM10_ESM.docx
Online Resource 10 Assessment of the most probable number of clusters (K) explaining the genetic structure of M. arenaria individuals (N = 604) from 22 samples genotyped with six microsatellite loci (all but Mar5) as detected with the ΔK graphical method developed by Evanno et al. (2005). a) Mean (±SD; N = 4) posterior probability of data; b) mean rate (±SD; N = 4) of change of the likelihood distribution; c) mean (±SD; N = 4) absolute values of the second-order rate of change of the likelihood distribution; d) ΔK. Arrows point to the greatest value of ΔK and the most probable number of clusters (K = 3) explaining the genetic structure in the dataset (DOCX 186 kb)
Rights and permissions
About this article
Cite this article
St-Onge, P., Sévigny, JM., Strasser, C. et al. Strong population differentiation of softshell clams (Mya arenaria) sampled across seven biogeographic marine ecoregions: possible selection and isolation by distance. Mar Biol 160, 1065–1081 (2013). https://doi.org/10.1007/s00227-012-2157-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2157-5