Abstract
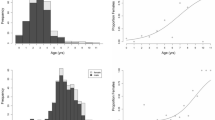
Thresholds to sexual maturity—either age or size—are critical life history parameters. Usually investigated in short-lived organisms, these thresholds and interactions among age, size, and growth are poorly known for long-lived species. A 34-year study of captive green turtles (Chelonia mydas) that followed individuals from hatching to beyond maturity provided an opportunity to evaluate these parameters in a long-lived species with late maturity. Age and size at maturity are best predicted by linear growth rate and mass growth rate, respectively. At maturity, resource allocation shifts from growth to reproductive output, regardless of nutrient availability or size at maturity. Although captive turtles reach maturity at younger ages than wild turtles, the extensive variation in captive turtles under similar conditions provides important insights into the variation that would exist in wild populations experiencing stochastic conditions. Variation in age/size at maturity should be incorporated into population models for conservation and management planning.





Similar content being viewed by others
References
Balazs GH, Chaloupka M (2004) Spatial and temporal variability in somatic growth of green sea turtles (Chelonia mydas) resident in the Hawaiian Archipelago. Mar Biol 145:1043–1059
Bell CDL, Parsons J, Austin TJ, Broderick AC, Ebanks-Petrie G, Godley BJ (2005) Some of them came home: the Cayman Turtle Farm headstarting project for the green turtle Chelonia mydas. Oryx 39:137–148
Bernardo J (1993) Determinants of maturation in animals. Trends Ecol Evol 8:166–173
Berner D, Blanckenhorn WU (2007) An ontogenetic perspective on the relationship between age and size at maturity. Funct Ecol 21:505–512
Bjorndal KA (1982) The consequences of herbivory for the life history pattern of the Caribbean green turtle. In: Bjorndal KA (ed) Biology and conservation of sea turtles. Smithsonian Institution Press, Washington, pp 111–116
Bjorndal KA (1985) Nutritional ecology of sea turtles. Copeia 1985:736–751
Bjorndal KA (1997) Foraging ecology and nutrition of sea turtles. In: Lutz PL, Musick JA (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 199–231
Bjorndal KA, Meylan AB, Turner BJ (1983) Sea turtles nesting at Melbourne Beach, Florida, 1. Size, growth and reproductive biology. Biol Conserv 26:65–77
Bjorndal KA, Bolten AB, Chaloupka MY (2000) Green turtle somatic growth model: evidence for density dependence. Ecol Appl 10:269–282
Bjorndal KA, Bolten AB, Dellinger T, Delgado C, Martins HR (2003) Compensatory growth in oceanic loggerhead sea turtles: response to a stochastic environment. Ecology 84:1237–1249
Bjorndal KA, Bowen BW, Chaloupka M, Crowder LB, Heppell SS, Jones CM, Lutcavage ME, Policansky D, Solow AR, Witherington BE (2011) From crisis to opportunity: better science needed for restoration in the Gulf of Mexico. Science 331:537–538
Bolten AB, Crowder LB, Dodd MG, MacPherson SL, Musick JA, Schroeder BA, Witherington BE, Long KJ, Snover ML (2011) Quantifying multiple threats to endangered species: an example from loggerhead sea turtles. Front Ecol Environ 9:295–301
Broderick AC, Glen F, Godley BJ, Hays GC (2003) Variation in reproductive output of marine turtles. J Exp Mar Biol Ecol 288:95–109
Broekhuizen N, Gurney WSC, Jones A, Bryant AD (1994) Modelling compensatory growth. Funct Ecol 8:770–782
Carr A, Goodman D (1970) Ecologic implications of size and growth in Chelonia. Copeia 1970:783–786
Casale P, Mazaris AD, Freggi D (2011) Estimation of age at maturity of loggerhead sea turtles Caretta caretta in the Mediterranean using length frequency data. Endanger Species Res 13:123–129
Chaloupka MY, Musick JA (1997) Age, growth, and population dynamics. In: Lutz PL, Musick JA (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 233–276
Chaloupka M, Limpus C, Miller J (2004) Green turtle somatic growth dynamics in a spatially disjunct Great Barrier Reef metapopulation. Coral Reefs 23:325–335
Chaloupka M, Bjorndal KA, Balazs GH, Bolten AB, Ehrhart LM, Limpus CJ, Suganuma H, Troëng S, Yamaguchi M (2008) Encouraging outlook for recovery of a once severely exploited marine megaherbivore. Global Ecol Biogeogr 17:297–304
Congdon JD, van Loben Sels RC (1991) Growth and body size variation in Blanding’s turtles (Emydoidea blandingi): relationships to reproduction. Can J Zool 69:239–245
Congdon JD, van Loben Sels RC (1993) Relationships of reproductive traits and body size with attainment of sexual maturity and age in Blanding’s turtles (Emydoidea blandingi). J Evol Biol 6:547–557
Czarnołęski M, Kozłowski J (1998) Do Bertalanffy’s growth curves result from optimal resource allocation? Ecol Lett 1:5–7
Day T, Taylor PD (1997) Von Bertalanffy’s growth equation should not be used to model age and size at maturity. Am Nat 149:381–393
Dieckmann U, Heino M (2007) Probabilistic reaction norms: their history, strengths, and limitations. Mar Ecol Prog Ser 335:253–269
Diez CE, van Dam RP (2002) Habitat effect on hawksbill turtle growth rates on feeding grounds at Mona and Monito Islands, Puerto Rico. Mar Ecol Prog Ser 234:301–309
Ernande B, Dieckmann U, Heino M (2004) Adaptive changes in harvested populations: plasticity and evolution of age and size at maturation. Proc R Soc B 271:415–423
Frazer NB, Ehrhart LM (1985) Preliminary growth models for green, Chelonia mydas, and loggerhead, Caretta caretta, turtles in the wild. Copeia 1985:73–79
Frazer NB, Ladner RC (1986) A growth curve for green sea turtles, Chelonia mydas, in the U.S. Virgin Islands, 1913–14. Copeia 1986:798–802
Goshe LR, Avens L, Scharf FS, Southwood AL (2010) Estimation of age at maturation and growth of Atlantic green turtles (Chelonia mydas) using skeletochronology. Mar Biol 157:1725–1740
Heithaus MR, Frid A, Wirsing AJ, Dill LM, Fourqurean JW, Burkholder D, Thomson J, Bejder L (2007) State-dependent risk-taking by green sea turtles mediates top-down effects of tiger shark intimidation in a marine ecosystem. J Anim Ecol 76:837–844
Heppell SS, Burchfield PM, Peña LJ (2007) Kemp’s ridley recovery: how far have we come, and where are we headed? In: Plotkin PT (ed) Biology and conservation of ridley sea turtles. Johns Hopkins University Press, Baltimore, pp 325–335
Hirth HF (1980) Some aspects of the nesting behavior and reproductive biology of sea turtles. Am Zool 20:507–523
Hirth HF (1997) Synopsis of the biological data on the green turtle Chelonia mydas (Linnaeus, 1758). U.S. Fish and Wildlife Service. Biol Rep 97:1–120
Kingsolver JG, Diamond SE, Seiter SA, Higgins JK (2012) Direct and indirect phenotypic selection on developmental trajectories in Manduca sexta. Funct Ecol 26:598–607
Kubis S, Chaloupka M, Ehrhart L, Bresette M (2009) Growth rates of juvenile green turtles Chelonia mydas from three ecologically distinct foraging habitats along the east central coast of Florida, USA. Mar Ecol Prog Ser 389:257–269
Limpus CJ (2009) A biological review of Australian Marine turtles. Queensland Environmental Protection Agency, Brisbane
Lutcavage ME, Plotkin P, Witherington B, Lutz PL (1997) Human impacts on sea turtle survival. In: Lutz PL, Musick JA (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 387–409
Madsen T, Shine R (2000) Silver spoons and snake body sizes: prey availability early in life influences long-term growth rates of free-ranging pythons. J Anim Ecol 69:952–958
McCauley SJ, Bjorndal KA (1999) Conservation implications of dietary dilution from debris ingestion: sublethal effects in post-hatchling loggerhead sea turtles. Conserv Biol 13:925–929
Mendonça MT (1981) Comparative growth rates of wild immature Chelonia mydas and Caretta caretta in Florida. J Herpetol 15:447–451
National Research Council (2010) Assessment of sea-turtle status and trends: integrating demography and abundance. National Academies Press, Washington, DC
Price ER, Wallace BP, Reina RD, Spotila JR, Paladino FV, Piedra R, Vélez E (2004) Size, growth, and reproductive output of adult female leatherback turtles Dermochelys coriacea. Endanger Species Res 5:1–8
Reich KJ, Bjorndal KA, Martínez del Rio C (2008) Effects of growth and tissue type on the kinetics of 13C and 15N incorporation in a rapidly growing ectotherm. Oecologia 155:651–663
Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Bull Fish Res Board Can 191:1–382
Roark AM, Bjorndal KA, Bolten AB (2009) Compensatory responses to food restriction in juvenile green turtles (Chelonia mydas). Ecology 90:2524–2534
Roff DA (2000) Trade-offs between growth and reproduction: an analysis of the quantitative genetic evidence. J Evol Biol 13:434–445
Roff DA (2002) Life history evolution. Sinauer, Sunderland
Scott R, Marsh R, Hays GC (2012) Life in the really slow lane: loggerhead sea turtles mature late relative to other reptiles. Funct Ecol 26:227–235
Shine R, Iverson JB (1995) Patterns of survival, growth and maturation in turtles. Oikos 72:343–348
Snover ML, Hohn AA, Crowder LB, Heppell SS (2007) Age and growth in Kemp’s ridley sea turtles: evidence from mark-recapture and skeletochronology. In: Plotkin PT (ed) Biology and conservation of ridley sea turtles. Johns Hopkins University Press, Baltimore, pp 89–105
Stamps JA (2007) Growth-mortality tradeoffs and ‘personality traits’ in animals. Ecol Lett 10:355–363
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stokes L, Wyneken J, Crowder LB, Marsh J (2006) The influence of temporal and spatial origin on size and early growth rates in captive loggerhead sea turtles (Caretta caretta) in the United States. Herpetol Conserv Biol 1:71–80
Turtle Expert Working Group (TEWG) (2009) An assessment of the loggerhead turtle population in the western North Atlantic Ocean. NOAA Technical Memorandum NMFS-SEFSC-575, p 142. http://www.sefsc.noaa.gov/turtles/TM_575_TEWG.pdf. Accessed 20 Oct 2012
Uusi-Heikkilä S, Kuparinen A, Wolter C, Meinelt T, O’Toole AC, Arlinghaus R (2011) Experimental assessment of the probabilistic maturation reaction norm: condition matters. Proc R Soc B 278:709–717
van Buskirk J, Crowder LB (1994) Life-history variation in marine turtles. Copeia 1994:66–81
Acknowledgments
This study was funded by the Disney Wildlife Conservation Fund. We are grateful to the staff of the Cayman Turtle Farm for their many years of work that made this study possible. We thank M. Chaloupka for constructive comments on the manuscript. All animal care was conducted incompliance with the Government of the Cayman Islands. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Lewison.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bjorndal, K.A., Parsons, J., Mustin, W. et al. Threshold to maturity in a long-lived reptile: interactions of age, size, and growth. Mar Biol 160, 607–616 (2013). https://doi.org/10.1007/s00227-012-2116-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2116-1