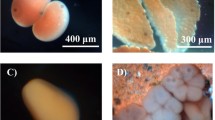
Abstract
Although recent studies have demonstrated that calcification in a wide range of marine organisms is profoundly affected by CO2-induced ocean acidification, the mechanism of this phenomenon is still unclear. To clarify the effects of ocean acidification on the calcification process at the molecular level, we evaluated the expression of three biomineralization-related genes in the sea urchin Hemicentrotus pulcherrimus exposed under control, 1,000, and 2,000 ppm CO2 from egg to pluteus larval stage. We found that the expression of the gene msp130, which is proposed to transport Ca2+ to the calcification site, is suppressed by increased CO2 at pluteus larval stage. Meanwhile, expression of the spicule protein matrix genes SM30 and SM50 was apparently not affected. The results suggest that the combined effects of ocean acidification on the expression of skeletogenesis-related genes as well as the change in seawater carbonate chemistry affect the biomineralization ability of sea urchins.


Similar content being viewed by others
References
Agatsuma Y (2007) Ecology of Hemicentrotus pulcherrimus, Pseudocentrotus depressus, and Anthocidaris crassispina. In: Lawrence JM (ed) Edible sea urchins: biology and ecology, pp 459–472
Akasaka K, Frudakis TN, Killian CE, George NC, Yamasu K, Khaner O, Wilt FH (1994) Genomic organization of a gene encoding the spicule matrix protein SM30 in the sea urchin Strongylocentrotus purpuratus. J Biol Chem 269:20592–20598
Beniash E, Aizenberg J, Addadi L, Weiner S (1997) Amorphous calcium carbonate transforms into calcite during sea urchin larval spicule growth. Proc R Soc Lond [Biol] 264:461–465
Byrne M (2011) Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr Mar Biol Annu Rev 49:1–42
Byrne M (2012) Global change ecotoxicology: identification of early life history bottlenecks in marine invertebrates, variable species responses and variable experimental approaches. Mar Environ Res 76:3–15
Caldeira K, Wickett ME (2003) Anthropogenic carbon and ocean pH. Nature 425:365
Clark D, Lamare M, Barker M (2009) Response of sea urchin pluteus larvae (Echinodermata: Echinoidea) to reduced seawater pH: a comparison among a tropical, temperate, and a polar species. Mar Biol 156:1125–1137
Doney SC, Fabry VJ, Feely RA, Kleypas JA (2009) Ocean acidification: the other CO2 problem. Annu Rev Mar Sci 1:169–192
Emlet RB (1982) Echinoderm calcite: a mechanical analysis from larval spicules. Biol Bull 163:264–275
Fabry V, Seibel BA, Feely RA, Orr JC (2008) Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J Mar Sci 65:414–432
Farach-Carson MC, Carson DD, Collier JL, Lennarz WJ, Park HR, Wright GC (1989) A calcium-binding, asparagines-linked oligosaccharide is involved in skeleton formation in the sea urchin embryo. J Cell Biol 109:1289–1299
Fuchikami T, Mitsunaga-Nakatsubo K, Amemiya S, Hosomi T, Watanabe T, Kurokawa D, Kataoka M, Harada Y, Satoh N, Kusunoki S, Takata K, Shimotori T, Yamamoto T, Sakamoto N, Shimada H, Akasaka K (2002) T-brain homologue (HpTb) is involved in the archenteron induction signals of micromere descendant cells in the sea urchin embryo. Development 129:5205–5216
Gattuso J-P, Frankignoulle M, Bourge I, Romaine S, Buddemeier RW (1998) Effect of calcium carbonate saturation of seawater on coral calcification. Glob Planet Change 18:37–46
Gattuso J-P, Allemand D, Frankignoulle M (1999) Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: a review on interactions and control by carbonate chemistry. Am Zool 39:160–183
Gazeau F, Quiblier C, Jansen JM, Gattuso J-P, Middelburg JJ, Heip CHR (2007) Impact of elevated CO2 on shellfish calcification. Geophys Res Let 34:L07603. doi:10.1029/2006GL028554
Guss K, Ettenson CA (1997) Skeletal morphogenesis in the sea urchin embryo: regulation of primary mesenchyme gene expression and skeletal rod growth by ectoderm-derived cues. Development 124:1899–1908
Hofmann GE, O’Donnell MJ, Todgham AE (2008) Using functional genomics to explore the effects of ocean acidification on calcifying marine organisms. Mar Ecol Prog Ser 373:219–225
Hofmann GE, Barry JB, Edmunds PJ, Gates RD, Hutchins DA, Klinger T, Sewell MA (2010) The effects of ocean acidification on calcifying organisms in marine ecosystems: an organism to ecosystem perspective. Annu Rev Ecol Evol Syst 41:127–147
Iglesias-Rodrigues MD, Halloran PR, Riskaby REM, Hall IR, Colmenero-Hidalgo E, Gittins JR, Green DRH, Tyrrell T, Gibbs SJ, von Dassaw P et al (2008) Phytoplankton calcification in a high-CO2 world. Science 320:336–340
IPCC 2007 (2007) The physical science basis. Working Group I Contribution to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge
Jury CP, Whitehead RF, Szmant AM (2010) Effects of variations in carbonate chemistry on the calcification rates of Madracis auretenra (=Madracis mirabilis sensu Wells, 1973): bicarbonate concentrations best predict calcification rates. Glob Change Biol 16:1632–1644
Katoh-Fukui Y, Noce T, Ueda T, Fujiwara Y, Hashimoto N, Higashinakagawa T, Killian CE, Livingston BT, Wilt FH, Benson SC, Sucov HM, Davidson EH (1991) The corrected structure of the SM50 spicule matrix protein of Strongylocentrotus purpuratus. Dev Biol 145:201–202
Killian C, Croker L, Wilt FH (2010) SpSM30 gene family expression patterns in embryonic and adult biomineralized tissues of the sea urchin, Strongylocentrotus purpuratus. Gene Exp Patterns 10:135–139
Kitajima T, Urakami H (2000) Differential distribution of spicule matrix proteins in the sea urchin embryo skeleton. Dev Growth Differ 42:295–306
Kleypas JA, Feely RA, Fabry VJ, Langdon C, Sabine CL, Robbins LL (2006) Impacts of ocean acidification on coral reefs and other marine calcifiers: a guide for future research, report of a workshop held 18–20 April 2005, St. Petersburg, FL, sponsored by NSF, NOAA, and the U.S. Geological Survey. http://www.isse.ucar.edu/florida/report/Ocean_acidification_res_guide_compressed.pdf
Kroeker KJ, Kordas RL, Crim RN, Singh GG (2010) Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol Lett 13:1419–1434
Kurihara H, Shirayama Y (2004) Effects of increased atmospheric CO2 on sea urchin early development. Mar Ecol Prog Ser 274:161–169
Leaf DS, Anstrom JA, Chin JE, Harkey MA, Showman RM, Raff RA (1987) Antibodies to a fusion protein identify a cDNA clone encoding msp130, a primary mesenchyme-specific cell surface protein of the sea urchin embryo. Dev Biol 121:29–40
Lewis E, Wallace DWR (1998) Program developed for CO2 system calculations. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge
Livingston BT, Killian CE, Wilt F, Cameron AC, Landrum MJ, Ermolaeva O, Sapojnikov V, Maglott DR, Buchanan AM, Ettensohn CA (2006) A genome-wide analysis of biomineralization-related proteins in the sea urchin Strongylocentrotus purpuratus. Dev Biol 300:335–348
Martin S, Richier S, Pedrotti M-L, Duppont S, Castejon C, Gerakis Y, Kerros M-E, Oberhänsli F, Teyssié J-L, Jeffree R, Gattuso J-P (2011) Early development and molecular plasticity in the Mediterranean sea urchin Paracentrotus lividus exposed to CO2-driven acidification. J Exp Biol 214:1357–1368
Melzner F, Gutowska MA, Langenbuch M, Dupont S, Lucassen M, Thorndyke MC, Bleich M, Pörtner HO (2009) Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences 6:2313–2331
O’Donnell MJ, Todgham AE, Sewell MA, Hammmond LM, Ruggiero K, Fangue NA, Zippay ML, Hofmann GE (2010) Ocean acidification alters skeletonesis and gene expression in larval sea urchins. Mar Ecol Prog Ser 398:157–171
Okazaki K (1975) Spicule formation by isolated micromeres of the sea urchin embryo. Am Zool 15:567–581
Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F et al (2005) Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437:681–686
Peled-Karmar M, Hamilton P, Wilt FH (2002) Spicule matrix protein LSM34 is essential for biomineralization of the sea urchin spicule. Exp Cell Res 272:56–61
Pennington JT, Emlet RB (1986) Ontogenatic and diel vertical migration of a planktonic echinoid larva, Dendraster excentricus (Eschscholtz): occurrence, causes, and probable consequences. J Exp Mar Biol Ecol 104:69–95
Pörtner H-O (2008) Ecosystem effects of ocean acidification in times of ocean warming: a physiologist’s view. Mar Ecol Prog Ser 373:203–217
Riebesell U, Zondervan I, Rost B, Tortell PD, Zeebe RE, Morel FMM (2000) Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 407:364–367
Ries JB, Cohen AL, McCorkie DC (2009) Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37:1131–1134
Rodolfo-Metalpa R, Martin S, Ferrier-Pagès C, Gattuso J-P (2010) Response of the temperate coral Cladocora caespitosa to mid- and long-term exposure to pCO2 and temperature levels projected for the year 2100 AD. Biogeosciences 7:289–300
Sheppard Brennand H, Soars N, Dworjanyn SA, Davis AR, Byrne M (2010) Impact of ocean warming and ocean acidification on larval development and calcification in the sea urchin Tripneustes gratilla. PLoS ONE 5:e11372. doi:10.1371/journal.pone.0011372
Stumpp M, Dupont S, Thorndyke MC, Melzner F (2011) CO2 induced seawater acidification impacts sea urchin larval development II: gene expression patterns in pluteus larvae. Comp Biochem Physiol A 160:320–330
Todgham AE, Hofmann GE (2009) Transcriptomic response of sea urchin larvae Strongylocentrotus purpuratus to CO2-driven seawater acidification. J Exp Biol 212:2579–2594
Widdicombe S, Spicer JI (2008) Predicting the impact of ocean acidification on benthic biodiversity: what can animal physiology tell us? J Exp Mar Biol Ecol 366:187–197
Wilt FH (2002) Biomineralization of spicules of sea urchin embryos. Zool Sci 19:253–261
Wilt FH, Killian CE, Livingston BT (2003) Development of calcareous skeletal elements in invertebrates. Development 71:237–250
Wood HL, Spicer JI, Widdicombe S (2008) Ocean acidification may increase calcification rates, but at a cost. Proc R Soc B. doi:10.1098/rspb.2008.0343
Yamazaki A, Kawabata R, Shiomi K, Amemiya S, Sawaguchi M, Mitsunaga-Nakatsubo K, Yamaguchi M (2005) The micro1 gene is necessary and sufficient for micromere differentiation and mid/hindgut-inducing activity in the sea urchin embryo. Dev Genes Evol 215:450–459
Zippay M, Hofmann G (2010) Effct of pH on gene expression and thermal tolerance of early life history stages of red abalone (Haliotis rufescens). J Shellfish Res 29:429–439
Acknowledgments
We thank Dr. James Davis Reimer for revising English of the manuscript. This study was partially supported by a grant from Japan Society for the Promotion of Science (#20710008) and by the Rising Star program of the University of the Ryukyus.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Byrne.
Rights and permissions
About this article
Cite this article
Kurihara, H., Takano, Y., Kurokawa, D. et al. Ocean acidification reduces biomineralization-related gene expression in the sea urchin, Hemicentrotus pulcherrimus . Mar Biol 159, 2819–2826 (2012). https://doi.org/10.1007/s00227-012-2043-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2043-1