Abstract
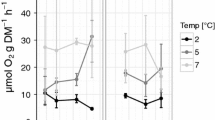
Many pteropod species in the eastern tropical North Pacific Ocean migrate vertically each day, transporting organic matter and respiratory carbon below the thermocline. These migrations take species into cold (15–10° C) hypoxic water (<20 μmol O2 kg−1) at depth. We measured the vertical distribution, oxygen consumption and ammonia excretion for seven species of pteropod, some of which migrate and some which remain in oxygenated surface waters throughout the day. Within the upper 200 m of the water column, changes in water temperature result in a ~60–75 % reduction in respiration for most species. All three species tested under hypoxic conditions responded to low O2 with an additional ~35–50 % reduction in respiratory rate. Combined, low temperature and hypoxia suppress the metabolic rate of pteropods by ~80–90 %. These results shed light on the ways in which expanding regions of hypoxia and surface ocean warming may impact pelagic ecology.






Similar content being viewed by others
References
Antezana T (2009) Species-specific patterns of diel migration into the oxygen minimum zone by euphausiids in the Humboldt Current ecosystem. Prog Oceanogr 83:228–236
Behrenfeld MJ, O’Malley RT, Siegel DA, McClain CR, Sarmiento JL, Feldman GC, Milligan AJ, Falkowski PG, Letelier RM, Boss ES (2006) Climate-driven trends in contemporary ocean productivity. Nature 444:752–755
Bopp L, Aumont O, Cadule P, Alvain S, Gehlen M (2005) Response of diatoms distribution to global warming and potential implications: a global model study. Geophys Res Lett 32:L19606
Boutilier RG (2001) Mechanisms of cell survival in hypoxia and hypothermia. J Exp Biol 204:3171–3181
Buesseler KO, Lamborg CH, Boyd PW, Lam PJ, Trull TW, Bidigare RR, Bishop JKB, Casciotti KL, Dehairs F, Elskens M (2007) Revisiting carbon flux through the ocean’s twilight zone. Science 316:567–570
Burd AB, Hansell DA, Steinberg DK, Anderson TR, Arístegui J, Baltar F, Beaupré SR, Buesseler KO, DeHairs F, Jackson GA (2010) Assessing the apparent imbalance between geochemical and biochemical indicators of meso-and bathypelagic biological activity: What the @ $! is wrong with present calculations of carbon budgets? Deep Sea Res (II Top Stud Oceanogr) 57:1557–1571
Childress JJ (1971) Respiratory adaptations to the oxygen minimum layer in the bathypelagic mysid Gnathophausia ingens. Biol Bull 141:109–121
Childress JJ, Mickel TJ (1980) A motion compensated shipboard precision balance system. Deep Sea Res 27:965–970
Childress JJ, Seibel BA (1998) Life at stable low oxygen levels: adaptations of animals to oceanic oxygen minimum layers. J Exp Biol 201:1223–1232
Childress JJ, Barnes AT, Quetin LB, Robison BH (1978) Thermally protecting cod ends for the recovery of living deep-sea animals. Deep Sea Res 25:419–422
Clarke KR (1993) Non parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E, Plymouth
Dam HG, Roman MR, Youngbluth MJ (1995) Downward export of respiratory carbon and dissolved inorganic nitrogen by diel-migrant mesozooplankton at the JGOFS Bermuda time-series station. Deep Sea Res (I Oceanogr Res Pap) 42:1187–1197
Fernández-Álamo MA, Färber-Lorda J (2006) Zooplankton and the oceanography of the Eastern Tropical Pacific: a review. Prog Oceanogr 69:318–359
Fiedler PC, Talley LD (2006) Hydrography of the eastern tropical Pacific: a review. Prog Oceanogr 69:143–180
Fraser KPP, Rogers AD (2007) Protein metabolism in marine animals: the underlying mechanism of growth. Adv Mar Biol 52:267–362
Gilmer RW (1974) Some aspects of feeding in thecosomatous pteropod molluscs. J Exp Mar Biol Ecol 15:127–144
Gilmer RW, Harbison GR (1986) Morphology and field behavior of pteropod molluscs: feeding methods in the families Cavoliniidae, Limacinidae and Peraclididae (Gastropoda: Thecosomata). Mar Biol 91:47–57
Glazier DS (2005) Beyond the ‘3/4-power law’: variation in the intra-and interspecific scaling of metabolic rate in animals. Biol Rev 80:611–662
Gracey AY, Troll JV, Somero GN (2001) Hypoxia-induced gene expression profiling in the euryoxic fish Gillichthys mirabilis. Proc Natl Acad Sci USA Biol Sci 98:1993
Guppy M, Withers P (1999) Metabolic depression in animals: physiological perspectives and biochemical generalizations. Biol Rev 74:1–40
Haddock SHD, Heine JN (2005) Scientific blue-water diving. California Sea Grant College Program, La Jolla
Hand SC, Hardewig I (1996) Downregulation of cellular metabolism during environmental stress: mechanisms and implications. Annu Rev Physiol 58:539–563
Hays GC (2003) A review of the adaptive significance and ecosystem consequences of zooplankton diel vertical migrations. Hydrobiologia 503:163–170
Hays GC, Harris RP, Head RN (1997) The vertical nitrogen flux caused by zooplankton diel vertical migration. Mar Ecol Prog Ser 160:57–62
Hochachka PW, Somero GN (2002) Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, New York
Hochachka PW, Buck LT, Doll CJ, Land SC (1996) Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci USA Biol Sci 93:9493
Honjo S, Manganini SJ, Krishfield RA, Francois R (2008) Particulate organic carbon fluxes to the ocean interior and factors controlling the biological pump: a synthesis of global sediment trap programs since 1983. Prog Oceanogr 76:217–285
Ivancic I, Degobbis D (1984) An optimal manual procedure for ammonia analysis in natural waters by the indophenol blue method. Water Res 18:1143–1147
Karstensen J, Stramma L, Visbeck M (2008) Oxygen minimum zones in the eastern tropical Atlantic and Pacific oceans. Prog Oceanogr 77:331–350
Longhurst AR, Harrison WG (1989) The biological pump: profiles of plankton production and consumption in the upper ocean. Prog Oceanogr 22:47–123
Maas AE, Elder LE, Dierssen HM, Seibel BA (2011) Metabolic response of Antarctic pteropods (Mollusca: Gastropoda) to food deprivation and regional productivity. Mar Ecol Prog Ser 441:129–139
Maas AE, Wishner KF, Seibel BA (2012) The metabolic response of pteropods to acidification reflects natural CO2-exposure in oxygen minimum zones. Biogeosciences 9:747–757
Marsh AG, Manahan DT (1999) A method for accurate measurements of the respiration rates of marine invertebrate embryos and larvae. Mar Ecol Prog Ser 184:1–10
Mayzaud P, Conover RJ (1988) O:N atomic ratio as a tool to describe zooplankton metabolism. Mar Ecol Prog Ser 45:289–302
Morrison JM, Codispoti LA, Smith SL, Wishner K, Flagg C, Gardner WD, Gaurin S, Naqvi SWA, Manghnani V, Prosperie L (1999) The oxygen minimum zone in the Arabian Sea during 1995. Deep Sea Res (II Top Stud Oceanogr) 46:1903–1931
Oschlies A, Schulz KG, Riebesell U, Schmittner A (2008) Simulated 21st century’s increase in oceanic suboxia by CO2-enhanced biotic carbon export. Global Biogeochem Cycles 22:GB4008
Paulmier A, Ruiz-Pino D (2009) Oxygen minimum zones (OMZs) in the modern ocean. Prog Oceanogr 80:113–128
Perry RI, Harding GC, Loder JW, Tremblay MJ, Sinclair MM, Drinkwater KF (1993) Zooplankton distributions at the Georges Bank frontal system: retention or dispersion? Cont Shelf Res 13:357–383
Pörtner HO (2010) Oxygen-and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213:881–893
Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322:690–692
Quetin LB, Childress JJ (1976) Respiratory adaptations of Pleuroncodes planipes to its environment off Baja California. Mar Biol 38:327–334
Robinson C, Steinberg DK, Anderson TR, Arístegui J, Carlson CA, Frost JR, Ghiglione JF, Hernández-León S, Jackson GA, Koppelmann R, Quéguiner B, Ragueneau O, Rassoulzadegan F, Robison BH, Tamburini C, Tanaka T, Wishner KF, Zhang J (2010) Mesopelagic zone ecology and biogeochemistry-a synthesis. Deep Sea Res (II Top Stud Oceanogr) 57:1504–1518
Rosa R, Seibel BA (2008) Synergistic effects of climate-related variables suggest future physiological impairment in a top oceanic predator. Proc Natl Acad Sci USA Biol Sci 105:20776–20780
Rosa R, Seibel BA (2010) Metabolic physiology of the Humboldt squid, Dosidicus gigas: implications for vertical migration in a pronounced oxygen minimum zone. Prog Oceanogr 86:72–80
Sanders NK, Childress JJ (1990) A comparison of the respiratory function of the haemocyanins of vertically migrating and non-migrating pelagic, deep-sea oplophorid shrimps. J Exp Biol 152:167–187
Sarmiento JL, Hughes TMC, Stouffer RJ, Manabe S (1998) Simulated response of the ocean carbon cycle to anthropogenic climate warming. Nature 393:245–249
Seibel BA (2007) On the depth and scale of metabolic rate variation: scaling of oxygen consumption rates and enzymatic activity in the Class Cephalopoda (Mollusca). J Exp Biol 210:1–11
Seibel BA (2011) Critical oxygen levels and metabolic suppression in oceanic oxygen minimum zones. J Exp Biol 214:326–336
Seibel BA, Drazen JC (2007) The rate of metabolism in marine animals: environmental constraints, ecological demands and energetic opportunities. Philos Trans R Soc Lond B Biol Sci 362:2061–2078
Seibel BA, Thuesen EV, Childress JJ, Gorodezky LA (1997) Decline in pelagic cephalopod metabolism with habitat depth reflects differences in locomotory efficiency. Biol Bull 192:262–278
Seibel BA, Chausson F, Lallier FH, Zal F, Childress JJ (1999) Vampire blood: respiratory physiology of the vampire squid (Cephalopoda: Vampyromorpha) in relation to the oxygen minimum layer. Exp Biol Online 4:1–10
Seibel BA, Dymowska A, Rosenthal J (2007) Metabolic temperature compensation and co-evolution of locomotory performance in pteropod moluscs. Integr Comp Biol 47:880–891
Smith KL Jr, Teal JM (1973) Temperature and pressure effects on respiration of thecosomatous pteropods. Deep-Sea Res Oceanogr Abstr 20:853–858
Steinberg DK, Carlson CA, Bates NR, Goldthwait SA, Madin LP, Michaels AF (2000) Zooplankton vertical migration and the active transport of dissolved organic and inorganic carbon in the Sargasso Sea. Deep Sea Res (I Oceanogr Res Pap) 47:137–158
Stramma L, Johnson GC, Sprintall J, Mohrholz V (2008) Expanding oxygen-minimum zones in the tropical oceans. Science 320:655–658
Stramma L, Schmidtko S, Levin LA, Johnson GC (2010) Ocean oxygen minima expansions and their biological impacts. Deep Sea Res (I Oceanogr Res Pap) 57:587–595
Svetlichny LS, Hubareva ES, Erkan F, Gucu AC (2000) Physiological and behavioral aspects of Calanus euxinus females (Copepoda: Calanoida) during vertical migration across temperature and oxygen gradients. Mar Biol 137:963–971
Vaquer-Sunyer R, Duarte CM (2011) Temperature effects on oxygen thresholds for hypoxia in marine benthic organisms. Global Chang Biol 17:1788–1797
Wiebe P, Morton A, Bradley A, Backus R, Craddock J, Barber V, Cowles T, Flierl G (1985) New development in the MOCNESS, an apparatus for sampling zooplankton and micronekton. Mar Biol 87:313–323
Wishner KF, Gowing MM, Gelfman C (2000) Living in suboxia: ecology of an Arabian Sea oxygen minimum zone copepod. Limnol Oceanogr 45:1576–1593
Wishner KF, Gelfman C, Gowing MM, Outram DM, Rapien M, Williams RL (2008) Vertical zonation and distributions of calanoid copepods through the lower oxycline of the Arabian Sea oxygen minimum zone. Prog Oceanogr 78:163–191
Wyrtki K (1962) The oxygen minima in relation to ocean circulation. Deep-Sea Res Oceanogr Abstr 9:11–23
Acknowledgments
We are grateful for the assistance of our divers R. Rosa, L. Elder, B. Phillips, C. Cass and P. Suprenand who provided us with research organisms. We would like to thank D. Outram for her assistance in collecting and compiling the distributional data as well as all the students who helped at sea and in the laboratory with the MOCNESS samples. We would like to acknowledge the hard work and dedication of the Captain and Crew of the R/V New Horizon, the R/V Seward Johnson and the R/V Knorr and to thank K. Daly for her organization of the ETP research expeditions. This work was funded by National Science Foundation grants to K. Wishner and B. Seibel (OCE—0526502 and OCE—0851043) and to K. Daly (OCE—0526545), the University of Rhode Island and the Rhode Island Experimental Program to Stimulate Competitive Research Fellowship program.
Ethical standards
The experiments were done in international waters, exempting them from legislation; however, experiments were conducted to comply with the current laws of the United States of America.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maas, A.E., Wishner, K.F. & Seibel, B.A. Metabolic suppression in thecosomatous pteropods as an effect of low temperature and hypoxia in the eastern tropical North Pacific. Mar Biol 159, 1955–1967 (2012). https://doi.org/10.1007/s00227-012-1982-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-1982-x