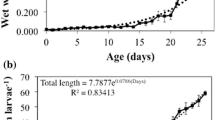
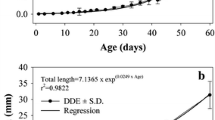
Abstract
The brown shrimp Crangon crangon is a key species in the coastal areas of the North Sea. It constitutes a significant food source for fishes. Simultaneously, it is an important predator on a wide range of invertebrates. C. crangon shows a variety of digestive enzymes that allow to utilizing a wide range of food items. The initial step of alimentary protein digestion, that is the degradation into peptides, is facilitated by set of endopeptidases which are expressed by the midgut gland. In crustaceans, these endopeptidases are often dominated by serine proteinases. C. crangon, however, predominantly express cysteine proteinases, while only some specimens show a highly variable pattern of serine proteinases. The composition of these serine endopeptidases was investigated using liquid chromatography, substrate gel electrophoresis and inhibitor assays. Distinctly elevated activities were present only in about 10% of the samples. When activity was detected, two peaks, one with tryptic activity and the other one with chymotryptic activity, could be separated by anionic exchange chromatography. Moreover, specimens with elevated tryptic activities often showed highly polymorphic patterns of endopeptidases after electrophoretic separation. Overall, 30 different bands of endopeptidases were identified. There was no similarity between animals from the same sampling sites, neither between animals of similar size, weight or nutritive state. The polymorphism of proteinase from the midgut gland seems to reflect the high adaptive potential of this species to variable trophic conditions in a continuously changing environment.










Similar content being viewed by others
References
Abdullah R, Shukor NA (1993) Isozyme variation between two closely related species Crangon crangon (L.) and Crangon allmanni Kinahan (Decapoda, Caridea). Crustaceana 64:114–121
Anderson M (2003) CAP: a FORTRAN computer program for canonical analysis of principal coordinates. Department of Statistics, University of Aukland
Anderson M, Willis T (2003) Canonical Analysis of Principal Coordinates: a useful method of constrained ordination for ecology. Ecology 84:511–525
Ansell A, Comely C, Robb L (1999) Distribution, movements and diet of macrocrustations on a Scottish sandy beach with particular reference to predation on juvenile fishes. Mar Ecol Prog Ser 176:115–130
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Ceccaldi HJ (1997) Anatomy and physiology of the digestive system. Advances in World Aquaculture. In: D’Abramo LR, Conklin DE, Akiyama DM (eds) Crustacean nutrition, vol 6. World Aquaculture Society, Baton Rouge, LA, pp 261–291
Christensen B, Jelnes J, Berg U (1978) Long-term isozyme variation in parthenogenetic polyploid forms of Lumbricillus lineatus (Enchytraeidae, Oligochaeta) in recently established environments. Hereditas 88:65–73
Clark J, Ilgen TL, Haire MF, Mykles DL (1991) Differential effects of oleic acid, sodium dodecyl sulfate, and protease inhibitors on the endopeptidase activities of the lobster multicatalytic proteinase. Comp Biochem Physiol 99B:413–417
Córdova-Murueta JH, García-Carreño FL (2002) Nutritive value of squid and hydrolized protein supplement in shrimp feed. Aquaculture 210:371–384
Dall W, Moriarty DJW (1983) Functional aspects of nutrition and digestion. In: Mantel LE (ed) The biology of crustacea, vol 5. Internal anatomy and physiological regulation. Academic Press, New York, pp 215–261
del Norte-Campos AGC, Temming A (1994) Daily activity, feeding and rations in gobies and brown shrimp in the northern Wadden Sea. Mar Ecol Prog Ser 115:41–53
Dittrich B (1992a) Comparative studies on the thermal properties of a trypsin-like protease in two hermit crabs. Helgol Meeresunters 46:45–52
Dittrich B (1992b) Life under extreme conditions: aspects of evolutionary adaptation to temperature in crustacean proteases. Polar Biol 12:269–274
Fernández I, Oliva M, Carrillo O, Van Wormhoudt A (1997) Digestive enzyme activities of Penaeus notialis during reproduction and moulting cycle. Comp Biochem Physiol 118A:1267–1271
Galgani F, Nagayama F (1988) Digestive proteolysis and digestive proteinases in deep sea crabs Geryon affinis and Chionecetes japonicus. Bull Jap Soc Sci Fish 54:983–987
García-Carreño FL, Dimes LE, Haard NF (1993) Substrate-gel electrophoresis for composition and molecular weight of proteinases or proteinaceous proteinase inhibitors. Anal Biochem 214:65–69
García-Carreño FL, Navarrete del Toro A, Ezquerra M (1997) Digestive shrimp proteases for evaluation of protein digestibility in vitro. I: Effect of protease inhibitors in protein ingredients. J Mar Biotechnol 5:36–40
Geiger R, Fritz H (1984) Trypsin. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 5. Verlag Chemie, Weinheim, pp 119–123
Gibson R, Yin M, Robb L (1995) The behavioural basis of predator-prey size relationships between shrimp (Crangon crangon) and juvenile plaice (Pleuronectes platessa). J Mar Biol Assoc UK 75:337–349
Kimura M, Ohta T (1971) Protein polymorphism as a phase of molecular evolution. Nature 229:467–469
Le Moullac G, Klein B, Sellos D, Van Wormhoudt A (1996) Adaptation of trypsin, chymotrypsin and α-amylase to casein level and protein source in Penaeus vannamei (Crustacea Decapoda). J Exp Mar Biol Ecol 208:107–125
Lloyd AJ, Yonge CM (1947) The biology of Crangon vulgaris L. in the bristol channel and severn estuary. J Mar Biol Assoc UK 26:626–661
Nelson K, Hedgecock D (1980) Enzyme polymorphism and adaptive strategy in the decapod crustacea. Am Nat 116:238–280
Oh C-W, Hartnoll RG, Nash RDM (2001) Feeding ecology of the common shrimp Crangon crangon in Port Erin Bay, Isle of Man, Irish Sea. Mar Ecol Prog Ser 214:211–223
Pihl L, Rosenberg R (1984) Food selection and consumption of the shrimp Crangon crangon in some shallow marine areas in western Sweden. Mar Ecol Prog Ser 15:159–168
Plagmann J (1939) Ernährungsbiologie der Garnele (Crangon vulgaris Fabr.). Helgol wiss Meeresunters 2:113–162
Rachor E, Schröder A (2003) Auswirkungen auf das Makrozoobenthos - Nutznießer und Geschädigte der Eutrophierung. In: Lozán JL, Rachor E, Reise K, Sündermann J, von Westernhagen H (eds) Warnsignale aus Nordsee und Wattenmeer. Eine aktuelle Umweltbilanz. Verlag Wissenschaftliche Auswertungen, Hamburg, pp 201–203
Rick W (1974a) Chymotrypsin. In: Bergmeyer HU (ed) Methoden der enzymatischen Analyse. Verlag Chemie GmbH, Weinheim, pp 1045–1051
Rick W (1974b) Trypsin. In: Bergmeyer HU (ed) Methoden der enzymatischen Analyse. Verlag Chemie GmbH, Weinheim, pp 1052–1063
Saborowski R, Sahling G, Navarrete del Toro MA, Walter I, García-Carreño FL (2004) Stability and effects of organic solvents on endopeptidases from the gastric fluid of the marine crab Cancer pagurus. J Mol Catal B Enzym 30:109–118
Sakharov IYu, Litvin FE, Mitkevitch OV, Samokhin GP, Bespalova ZD (1994) Substrate specificity of collagenolytic proteases from the king crab Paralithodes camtschatica. Comp Biochem Physiol 107B:411–417
Teschke M, Saborowski R (2005) Cysteine proteinases substitute for serine proteinases in the midgut glands of Crangon crangon and Crangon allmani (Decapoda: Caridea). J Exp Mar Biol Ecol 316:213–229
Wahle RA (1985) The feeding ecology of Crangon franciscorum and Crangon nigricauda in San Francisco Bay, California. J Crust Biol 5:311–326
Wilcox JR, Jeffries HP (1974) Feeding habitats of the sand shrimp Crangon septemspinosa. Biol Bull 146:424–434
Acknowledgments
We gratefully acknowledge the excellent support of the crews of R/V Aade and R/V Uthörn. Dr. Jennifer Dannheim created the ArgGis 9-map of the German Bight.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. O. Pörtner.
Rights and permissions
About this article
Cite this article
Saborowski, R., Schatte, J. & Gimenez, L. Catalytic properties and polymorphism of serine endopeptidases from the midgut gland of the brown shrimp Crangon crangon (Decapoda, Caridea). Mar Biol 159, 1107–1118 (2012). https://doi.org/10.1007/s00227-012-1890-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-1890-0