Abstract
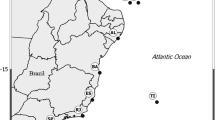
The zoanthid family Neozoanthidae (Anthozoa: Hexacorallia: Zoantharia) was described in 1973 from Madagascar as a monogeneric and monotypic taxon, and never reported again in literature. In 2008–2010, numerous zoanthid specimens fitting the morphological description of Neozoanthus were collected in the Ryukyu Islands, Okinawa, Japan, and the Great Barrier Reef (GBR), Australia. Utilizing these specimens, this study re-examines the phylogenetic position of Neozoanthidae and analyzes the evolutionary history of sand incrustation in zoanthids through phylogenetic and ancestral state reconstruction analyses. Specimens were colonial, partially incrusted with large, irregular sand and debris, zooxanthellate, and found from the intertidal zone to depths of approximately 30 m. Phylogenetic results utilizing mitochondrial 16S ribosomal DNA and cytochrome oxidase subunit I sequences show the presence of two Neozoanthus species groups, one each from Japan and the GBR. Unexpectedly, the molecular results also show Neozoanthus to be very closely related to the genus Isaurus, which as a member of the family Zoanthidae, is not sand incrusted. These results suggest that during evolution zoanthids can acquire and lose the ability to incrust sand with relative rapidity.




Similar content being viewed by others
References
Burnett WJ, Benzie JAH, Beardmore JA, Ryland JS (1997) Zoanthids (Anthozoa, Hexacorallia) from the Great Barrier Reef and Torres Strait, Australia: systematics, evolution and a key to species. Coral Reef 16:55–68
Ezaki Y (1997) The Permain coral Numidiaphyllum: new insights into anthozoans phylogeny and Triassic scleractinian origins. J Paleontol 40:1–14
Fautin DG (2009) Hexacorallians of the world. http://geoportal.kgs.ku.edu/hexacoral/anemone2/index.cfm
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotech 3:294–299
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Haywick DW, Mueller EM (1997) Sediment retention in incrusting Palythoa spp.—a biological twist to a geological process. Coral Reef 16:39–46
Herberts C (1972) Etude systématique de quelques zoanthaires tempérés et tropicaux. Tethys Supp 3:69–156 (in French)
Hirose M, Obuchi M, Irei Y, Fujii T, Reimer JD (in press) Timing of spawning and early development of Palythoa tuberculosa (Anthozoa, Zoantharia, Sphenopidae) in Okinawa, Japan. Biol Bull
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kurihara H (2008) Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar Ecol Prog Ser 373:275–284
Maddison WP, Maddison DR (2010) Mesquite: a modular system for evolutionary analysis. Version 2.74. Available from: http://mesquiteproject.org
Marshall CR, Raff EC, Raff RA (1994) Dollo’s law and the death and resurrection of genes. Proc Natl Acad Sci USA 91:12283–12287
Reimer JD, Ono S, Takishita K, Fujiwara Y, Tsukahara J (2004) Reconsidering Zoanthus spp. diversity: molecular evidence of conspecifity within four previously presumed species. Zool Sci 21:517–525
Reimer JD, Takishita K, Maruyama T (2006) Molecular identification of symbiotic dinoflagellates (Symbiodinium spp.) from Palythoa spp. (Anthozoa: Hexacorallia) in Japan. Coral Reef 25:521–527
Reimer JD, Hirano S, Fujiwara Y, Sinniger F, Maruyama T (2007) Morphological and molecular characterization of Abyssoanthus nankaiensis, a new family, new genus and new species of deep-sea zoanthid (Anthozoa: Hexacorallia: Zoantharia) from a northwest Pacific methane cold seep. Invertebr Systemat 21:255–262
Reimer JD, Ono S, Tsukahara J, Iwase F (2008) Molecular characterization of the zoanthid genus Isaurus (Anthozoa: Hexacorallia) and its zooxanthellae (Symbiodinium spp). Mar Biol 153:351–363
Reimer JD, Ishikawa SA, Hirose M (2010a) New records and molecular characterization of Acrozoanthus (Cnidaria: Anthozoa: Zoanthidae) from Taiwan. Mar Biodiv. doi:10.1007/s12526-010-0069-5
Reimer JD, Nakachi S, Hirose M, Hirose E, Hashiguchi S (2010b) Using hydrofluoric acid for morphological investigations of zoanthids (Cnidaria: Anthozoa): a critical assessment of methodology and necessity. Mar Biotech 12:605–617
Rodriguez F, Oliver JL, Marin A, Medina JR (1990) The general stochiatic model of nucleotide substitution. J Theor Biol 142:485–501
Ronquist F, Huelsenbeck JP (2003) Bayesian phylogenetic inference under mixed models. Bioinformatics (Oxf) 19:1572–1574
Ryland JS (1997) Budding in Acrozoanthus Saville-Kent, 1893 (Anthozoa: Zoanthidea). In: den Hartog JC (ed) Proceedings of the 6th international conference of coelenterate biology. Nationaal Natuurhistorisch Museum, Leiden, pp 423–428
Sinniger F, Pawlowski J (2009) The partial mitochondrial genome of Leiopathes glaberrima (Hexacorallia: Antipatharia) and the first report of the presence of an intron in COI in black corals. Galaxea 11:21–26
Sinniger F, Montoya-Burgos JI, Chevaldonné P, Pawlowski J (2005) Phylogeny of the order Zoantharia (Anthozoa, Hexacorallia) based on the mitochondrial ribosomal genes. Mar Biol 147:1121–1128
Sinniger F, Reimer JD, Pawlowski J (2010) The parazoanthidae DNA taxonomy: description of two new genera. Mar Biodivers 40:57–70
Acknowledgments
The Census of Coral Reef Ecosystems (CReefs) Australia Project is sponsored by BHP Billiton in partnership with the Great Barrier Reef Foundation and the Australian Institute of Marine Science (AIMS). CReefs is a field program of the Census of Marine Life. In Japan, all MISE laboratory members (2008–2010) are thanked for field help, as are Dr. Ryuji Asami (Rising Star Program) and Ryuji Suzuki (Marine Service Kamui, Tokunoshima). CReefs Australia project leader Dr. Julian Caley, field logistics manager Shawn Smith (both AIMS) and all Heron Island 2009 CReefs survey participants are thanked for their help in specimen collection. JDR was partially supported by the Rising Star Program at the University of the Ryukyus, and a grant from the Japan Society for the Promotion of Science (“Wakate B” # 2170088). Specimens from Australia were collected under Great Barrier Reef Marine Park Authority permit # G32313.1 and Queensland Fisheries permit # 95152. Two anonymous reviewers’ comments greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Riginos.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Scanning electron microscope image of a cross-section of specimen MISE 539 showing incrusted sponge spicules (ss) with relatively intact mesoglea (m) and remains of locations of other embedded materials (rough areas surrounding mesoglea). Scale = 200 μm. (TIFF 758 kb)
Fig. S2
Maximum likelihood (ML) tree of cytochrome oxidase subunit 1 (COI) sequences for zoanthid specimens. Values at branches represent ML probabilities (>50%) and Bayesian posterior probabilites (>0.50), respectively. Sequences newly obtained in this study in bold. Sequences/species names from previous studies in regular font with GenBank Accession Number. For specimen information see Table S1. (EPS 365 kb)
Rights and permissions
About this article
Cite this article
Reimer, J.D., Hirose, M., Irei, Y. et al. The sands of time: rediscovery of the genus Neozoanthus (Cnidaria: Hexacorallia) and evolutionary aspects of sand incrustation in brachycnemic zoanthids. Mar Biol 158, 983–993 (2011). https://doi.org/10.1007/s00227-011-1624-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-011-1624-8