Abstract
Carnivorous zooplankton is a key element to the energy transfer through the arctic food web, linking lipid rich herbivores to the top predators. We investigated the growth and lipid dynamic of the Arctic pelagic amphipod Themisto libellula in Kongsfjorden (Svalbard, 79°N) from May to October 2007. Additional samplings were performed in spring and summer 2006 and further north in Rijpfjorden (80°N), in September 2006 and 2007. In Kongsfjorden, the first free-swimming stages (3 mm) appeared early May and reached their adult length (25 mm), in October. During their first year, they grew according to a Von Bertalanffy model and most probably constituted a single cohort. Juveniles had the highest growth rate (0.19 mm day−1) and revealed relatively low total lipid (TL) content (about 2.5% wet weight (WW)) with phospholipids as the major lipid class. Sub-adults showed a distinct decrease of growth rates which coincided with the increase of neutral lipid storage, reflecting a switch in energy allocation, from somatic growth to lipid storage. Indeed wax esters (WE) increased up to 48.5% TL on average in adults in 2006 while triacylglycerols (TAG) remained almost constant below 25.2% TL. The absence of lipid accumulation (in disproportion of the weight) in 2007 could be explained by a higher metabolism of T. libellula or preys of lower quality. In Rijpfjorden, adults in their second year continued accumulating lipid (up to 10% WW) with high and similar proportions of both lipid classes, WE and TAG. We highlighted that T. libellula exhibited a variable lipid metabolism along its life cycle depending on its physiological needs and environmental conditions.
Similar content being viewed by others
Introduction
Arctic pelagic ecosystems are highly influenced by annual light cycle and presence of sea ice (Falk-Petersen et al. 2007). Consequently phytoplankton blooms are usually intense and restricted to a short period occurring between April and September, depending on the latitude. To cope with this high variability in food supply, zooplankton has developed different adaptations either in terms of metabolism, life cycle or feeding habits (Lee et al. 2006). Among these adaptations, polar organisms have the noticeable capacity to store large amounts of energy as lipids during the productive season and to use them either for reproduction or survival during periods of food paucity. Thus fluxes within these ecosystems are commonly considered to be based on the amount of lipids transferred along the food chain (Falk-Petersen et al. 1990).
Carnivorous species are usually less lipid-rich than herbivorous species because of their capacity to find food the year round. This is probably the reason why relatively few studies focused on lipids of these species. Nevertheless they contribute to the lipid transfer between rich herbivorous zooplankton and top predators. A good knowledge of their lipid composition and dynamic is thus essential to understand ecosystem functioning and energy transfers through the food web. Among these carnivorous species, the pelagic amphipod Themisto libellula Lichtenstein in Mandt 1822 (Crustacea: Amphipoda: Hyperiidae) is highly abundant and widely distributed in the Arctic Ocean (Dunbar 1957; Dalpadado et al. 2001; Dalpadado 2002). This amphipod could contribute significantly to energy transfers as it feeds mainly on copepods (Auel et al. 2002; Dalpadado et al. 2008; Marion et al. 2008; Noyon et al. 2009) and because it represents a major food source for higher predators such as fish (Hop et al. 1997; Dalpadado and Bogstad 2004), seals (Nilssen et al. 1995; Falk-Petersen et al. 2004; Haug et al. 2004) and seabirds (Lønne and Gabrielsen 1992).
This species has a life cycle of 1–3 years (Dunbar 1957; Wing 1976; Koszteyn et al. 1995; Dalpadado 2002; Dale et al. 2006). Eggs and the three first post-hatched stages are kept in the female marsupium pouch (Dunbar 1946; Percy 1993b). Then the first free-swimming juveniles (2–4 mm) are released in spring and grow until the beginning of the next winter (Percy 1993b; Dale et al. 2006). They can reach about 19 mm in length which corresponds to their sexual maturation (Dunbar 1946; Bowman 1960; Percy 1993b; Dale et al. 2006), consequently they can already reproduce during their first winter. Only two studies analysed the lipid composition of T. libellula. One was conducted on adults caught in the Fram Strait in summer (Auel et al. 2002) and the other one studied the lipid content of juveniles from the marginal ice zone of the Barents Sea in June (Scott et al. 1999). They revealed intermediate level of total lipids (22.3 ± 7% and 38.9 ± 7.9% dry weight (DW) for males and juveniles, respectively) compared to lipid rich copepods (Lee et al. 2006), with either wax esters (WE) or phospholipids (PL) as the dominant lipid classes. These punctual studies did not allow to determine their lipid dynamics although this is an important matter of interest to understand their lipid metabolism and adaptive strategies.
Therefore we propose in the present paper to fill up this gap by following in detail the lipid class composition along growth from the first free-swimming stages to the adult stages. Investigations were conducted from May to October 2007 in Kongsfjorden, a fjord with the particularity to entrap plankton due to an eddy in the outer part of the fjord (Basedow et al. 2004) which should make the study of a single cohort easier. For comparison, additional samples collected in the same fjord the previous year and in a fjord located further north, Rijpfjorden, were also analysed.
Materials and methods
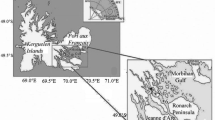
The main sampling station was located in the middle part of Kongsfjorden, a fjord located on the west coast of Svalbard archipelago (78°57′N, 11°56′E, Fig. 1) and another additional sampling station was situated in Rijpfjorden, on the northern part of Svalbard archipelago (80°17′N, 22°15′E, Fig. 1).
Primary and secondary biomass measurements
Chlorophyll a concentration as a proxy of phytoplankton biomass and mesozooplankton biomass were monitored weekly from May to September 2007 in Kongsfjorden. Chlorophyll a was measured using a Seapoint Chlorophyll Fluorometer (Sea-point Sensors, Inc., Exeter, USA) with one measurement per second corresponding to a vertical accuracy of 0.2–0.7 m. Then using an average value for every 5 m, chlorophyll a concentration was integrated over the water column from 180 m depth to the surface. Mesozooplankton biomass was obtained with a WP2 net (0.25 m² opening and 200 μm mesh size) towed vertically from 200 m to the surface. Samples were filtered on a pre-weight 200 μm mesh, rinsed with ammonium-formiate, placed in a drying oven for 48 h at 60°C and then re-weighted.
Growth of Themisto libellula
Once a week, from May to beginning of October 2007, a WP3 net (1 m² opening and 1 mm mesh size) was towed obliquely from 200 m to the surface in Kongsfjorden. Samples were directly fixed with 4% formalin (v/v final concentration).
Each collected T. libellula were measured using a binocular microscope provided with MotiCam 1,000 digital camera and its associated software Motic Image Plus (capture resolution 640 · 512 pixels). Length measurements were taken on the dorsal part of the organisms from the tip of the head to the longest uropod (Dunbar 1957) with ImageJ 1.40 software (public domain software developed by W.S. Rasband, U.S. National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/, 1997–2007). For each sample, all specimens were measured, except the 13th and 27th of June. Due to the large number of Themisto caught these 2 days, a sub-sample of 250 individuals was chosen randomly and measured. For each sampling date, the mean length was calculated and the preliminary plot revealed a sigmoid general pattern. Data were then fitted to the 3 following growth models: Gompertz, Logistic and Von Bertalanffy using Systat 11 (Systat software). The Von Bertalanffy model revealed the smaller sum square difference between predicted and measured values and thus was selected. This model has the following form: \( L_{t} = Lm \cdot ( 1- e^{{ - g \cdot (t - t_{ 0} )}} ) \), with L t as T. libellula length at age t, Lm the maximum estimated length, g the specific growth rate and t 0 the theoretical date of birth. These four coefficients were determined in order to minimise sum of squares. Growth rates (day−1) were estimated by the first derivative function \( {{{{\text{d}}L}}/{{{\text{d}}t}}} = g \cdot (Lm - L_{t} ) \).
Weight and lipid analyses
Organisms for lipid analyses were caught with double oblique WP3 nets and/or with Methot Isaac Kidd (MIK, 3.14 m² opening, 1.5 mm mesh size and 500 μm on the last 1.5 m of net).
The main set of data originated from May to October 2007 in Kongsfjorden. Additional samples were taken in Kongsfjorden from May to August 2006 in order to compare 2 years and in Rijpfjorden in September 2006 and October 2007 to catch larger adults of T. libellula which were absent in Kongsfjorden in 2006 and 2007. Sampling dates are summarized in Table 1.
Immediately after the catch, organisms were sorted and frozen alive at −80°C for conservation. Before lipid extraction, length (L) and weight wet (WW) of each organisms were measured as fast as possible on crushed ice to avoid lipid degradation. Organisms were stretched on a millimetre paper and total length from the tip of the head to the end of the uropod was measured to the nearest millimetre. According to Dale et al. (2006) classification, stages of T. libellula were determined based on their total length as juveniles (<12 mm), immature sub-adults (13–19 mm) and mature adults (>20 mm). We decided not to sex the organisms due to methodological limitations. Indeed males of T. libellula are determined by segmentation and length of their antenna but they are often broken during catch or conservation at −80°C. Females are characterised by presence of oostegites but time to find them is often too long and thus risks of lipid degradation appeared. Based on their length, organisms were grouped together to have enough lipid mass to perform all the analyses (from groups of 20 individuals for 3 mm organisms down to individual extraction for 14 mm organisms and larger).
Bligh and Dyer method (1959) was used for lipid extraction with a potter homogenizer (glass/Teflon) or a larger crusher depending on the organism length. Organisms were homogenized at 0°C in the solvent mixture (Chloroform:methanol:NaCl 1/2/0.8, v/v/v) then body fragments were separated from the solvent and re-extracted twice. After evaporation of the solvents, dry extracted lipids were weighted in tarred vials on a microscale (Sartorius). Samples were conserved at −80°C under nitrogen atmosphere to avoid lipid degradation, until further analyses. Total lipid content (TL) was expressed in mg ind−1 or as a percentage of wet weight (%WW).
Lipid classes were quantified using an Iatroscan MK V TH10 thin-layer chromatography-flame-ionization detector analyser (TLC–FID, Ackman 1981). Aliquots of TL (1 μl) were deposited in duplicates on quartz chromatorods SIII covered with silica. Neutral lipids were separated using two different migration systems with the following solvents systems: hexane:benzene:formic acid 80/20/1 (v/v/v), followed by hexane:diethyl ether:formic acid 97/3/1.5 (v/v/v).
Results
Themisto libellula growth and environmental conditions in Kongsfjorden in 2007
In Kongsfjorden, the first specimens of T. libellula sampled early May 2007 had a mean length (L) of 5 mm and reached more than 20 mm in October. The growth dynamic followed a Von Bertallanffy model equation: \( L_{t} = 2 6. 7 6\cdot ( 1- e^{ - 0. 0 0 8 5\cdot (t + 1 4. 6 )} ) \) (Fig. 2). Growth rates of T. libellula decreased rapidly between May and early July from 0.19 down to 0.10 mm day−1 (Fig. 3). From July, low and relatively constant growth rates were measured with minimum values around 0.03 mm day−1 recorded early October.
Phytoplankton bloom started mid-May with 250 mg chl a m−2 and persisted for 2–3 weeks (Fig. 3). Mesozooplankton biomass increased rapidly with only 1 week delayed from the onset of the spring bloom. For almost a month and a half, mesozooplankton biomass remained relatively high with 15–20 g m−2 and decreased mid-July to values lower than 10 g m−2 (Fig. 3).
Wet weight of the different developmental stages
Morphological characteristics of the different stages from both studied fjords and years are summarized in Table 2. Mean length and WW for each stage were quiet similar in Kongsfjorden between the 2 years but adults from Rijpfjorden were significantly larger (Student Test, p < 0.0001) than in Kongsfjorden. Significant relationships between length and WW were found in both fjords for both years (Table 2). In addition, all data collected in this study fitted the same general allometric relationship (WW = 0.024· L 2.75, Fig. 4).
Allometric relationship between wet weight (mg) and length (mm) of Themisto libellula collected in Kongsfjorden and Rijpfjorden, in 2006 and 2007 (the general allometric relationship is the following: \( WW = 0. 0 2 4\cdot L^{ 2. 7 5} \), allometric relationships for each fjords and years are detailed in Table 2)
Total lipid content
Total lipid content of T. libellula collected in Kongsfjorden and Rijpfjorden are described in Table 3. In Kongsfjorden, juvenile lipid content was similar during both years with values ranging from 0.02 to 0.61 mg ind−1 corresponding to 1.33–4.21% WW. Concerning sub-adults, their lipid content reached in 2006 a mean value of 2 mg ind−1, corresponding to 3.85% WW whereas in 2007 lipid content per individual was half the value found the previous year (1 mg ind−1) and represented only 2.02% WW. This value was even lower than what was found in juveniles of the same year. An even more pronounced difference between years was observed for the adults caught in Kongsfjorden since their total lipid content reached a mean of 7.26 mg ind−1 in 2006 and only 3.15 mg ind−1 in 2007. As described in Fig. 5, lipid content appeared to increase with length during both years (2006: r² = 0.92, p < 0.0001; 2007: r² = 0.76, p < 0.0001, panel a) but accumulation of lipids, i.e. increase of lipids in disproportion of their WW, was only observed in 2006 (r² = 0.46, p < 0.001, panel b). In comparison, adults caught in Rijpfjorden reached the highest lipid content recorded in our study with 29.74 and 42.93 mg ind−1 in 2006 and 2007 respectively, which represented approximately 10–11% WW.
Total lipid content (TL) of Themisto libellula as a function of length, from Kongsfjorden in 2006 (white triangle and solid line) and 2007 (black triangle and dashed line). On the left panel (a), TL is expressed per individual (mg ind−1) and on the right panel (b), TL is expressed in percentage of wet weight (% WW). Data fitted the following equations: a TL2006 = −0.20 + 0.17 10−3 L 3.41, r² = 0.92, p < 0.0001; TL2007 = 0.17 + 4.25 10−6 L 4. 35, r² = 0.76, p < 0.0001; b TL2006 = 3.044 + 3.84 10−7 L 4. 99, r² = 0.46, p < 0.001; no significant relationship was found in 2007 between TL expressed in % WW and length
Lipid composition
Percentages of each lipid classes are described in Table 4. The 3 major lipid classes were phospholipids (PL), triacylglycerols (TAG) and wax esters (WE). Proportion of PL was about 40–49% TL for juveniles in 2006 and 2007. This percentage decreased with length down to 17.3% TL in 2006 in Kongsfjorden and was about 10% TL for the adults from Rijpfjorden. On the other hand, it stayed the major lipid class for sub-adults (44% TL) and adults (38% TL) caught in Kongsfjorden in 2007. The quantity of PL per individual increased exponentially with length (2006: r = 0.91, p < 0.0001 and 2007: r = 0.83, p < 0.0001, Fig. 6) without any significant difference between the 2 years (p < 0.0001). Hence the organisms caught in 2006 and 2007 in Kongsfjorden might mostly differ by their quantity of neutral lipid (WE and/or TAG) accumulated.
WE proportion of juveniles in 2006 reached 25.6% TL whereas much lower levels were found in 2007 accounted for only 8.4% TL. However during both years, WE proportion increased along T. libellula growth. In 2006, high percentages of WE were found in sub-adults (41.5 ± 7.3% TL) and adults (48.5 5 ± 8.4% TL) compared to juveniles, whereas in 2007, only half of these values were measured. Relatively high levels of WE were also found in large adults from Rijpfjorden (34.0 ± 10.1% TL and 40.2 ± 8.3% TL in 2006 and 2007, respectively).
Concerning TAG, proportions in Kongsfjorden were relatively constant with level about 20% TL in 2006 and 2007, whatever the stage considered. The highest percentages of TAG were recorded in adults from Rijpfjorden with 43.6 ± 11.2% TL and 35.5 ± 9.1% TL in 2006 and 2007, respectively.
Hydrocarbon (HC), free fatty acids (FFA), sterols (ST) and diglycerides (DG) were present in low amount. ST and DG were relatively constant with ca. 5–7% and 2–5% TL on average, respectively. Globally FFA never exceeded 1% TL or were absent except in Kongsfjorden in 2007 where they reached 3% TL. Unusual lipids, as free fatty alcohols and diacylglycerol ethers (DAGE) were observed during analyses but their contributions were too low to be quantified.
Discussion
Growth and total lipid
During the seasonal survey of 2007, the first juveniles of 5 mm length appeared early May in Kongsfjorden and then grew up to 20–25 mm in October, as it has also been reported in earlier studies (Percy 1993b; Koszteyn et al. 1995; Dalpadado 2002; Dale et al. 2006). According to a Van Bertalanffy model, juvenile stages (5–13 mm) caught in May and June had the highest growth rates of 0.19–0.10 mm day−1 as Percy (1993b) measured it in Frobisher Bay. Considering our results and those from Basedow et al. (2004) about the maintenance of zooplankton in the inner part of the fjord, we could safely consider that a single cohort of T. libellula was present in Kongsfjorden in 2007. In addition, extending our dataset using a minimal growth rate of 0.05 mm day−1 in wintertime as suggested by Percy (1993b), the second year generation should reach about 30 mm in April of the next year which agrees with size class found in April 2002 in Kongsfjorden by Dale et al. (2006). Thus although we did not catch adult females in spring inside the fjord, we might consider that hatching of T. libellula could have occurred inside Kongsfjorden, just before the sampling started and that the fjord could be considered as a nursery for this species as already suggested for another species Themisto gaudichaudii (Labat et al. 2005).
Juveniles had a relatively low and similar total lipid content during both years (2.7 and 2.3% WW in 2006 and 2007 respectively) and their main lipid class was PL, like Scott et al. (1999) recorded. Considering that PL are the main constituent of membranes and that juveniles had high growth rates, it seems that as other early developmental stages of zooplankton, most of their energy was invested in somatic growth and not to build up lipid storage (Falk-Petersen et al. 1981; Kattner and Krause 1987; Kattner et al. 1994; Hagen et al. 2001). It can also be noticed that their lipid content did not decrease in the course of the study suggesting that growth was mainly sustained by dietary input and not by internal reserves. Moreover their low lipid level suggested a limited starvation capacity which implied a continuous food intake. Data from gut content analyses and fatty acid trophic markers (Noyon 2009) revealed that early juveniles fed on both phytoplankton and zooplankton. Consequently the timing of the release of the first free swimming specimens, just before the onset of the spring bloom, should be seen as an advantage enabling juveniles to take profit, first, of the phytoplankton bloom and then of the mesozooplankton bloom.
From July to September only sub-adults and adults of T. libellula were present in Kongsfjorden. During this period, their growth rates slowed down compared to juveniles while their lipid content increased. Lower growth rates for adults are very common in zooplanktonic species and are usually attributed to a shift of energy investment from growth towards lipid storage and/or sexual development (Kattner et al. 1994; Hagen et al. 2001). Interestingly, we showed that adults and sub-adults significantly accumulated lipids in 2006 whereas no accumulation was detectable in 2007. Two hypotheses might explain this difference. Water temperature in Kongsfjorden was exceptionally warm in 2007 compared to 2006, most probably due to an increase of Atlantic influence (Ledang et al. personal communication). Increasing sea water temperature induces an increase of the metabolism and thus of the energy expenditure. Hence in Kongsfjorden in 2007, most of the energy input could have been used by T. libellula to sustain a higher basal metabolism rather than to accumulate lipids. The other hypothesis is an indirect effect of the Atlantic inflow. Atlantic water advections bring in Kongsfjorden Atlantic species inducing a modification of the available preys for T. libellula (Basedow et al. 2004; Willis et al. 2006; Willis et al. 2008). As Atlantic species are less lipid rich than typical Arctic species, these advection events might lead to a decrease in lipid fluxes towards higher trophic levels (Stempniewicz et al. 2007; Kattner and Hagen 2008). The difference of lipid content between the two preferred preys of T. libellula, Calanus glacialis and C. finmarchicus (Noyon et al. 2009), can illustrate this hypothesis. Indeed lipid content of C. glacialis, the most Arctic species among those two, is about 0.05 mg ind−1 for the copepodite stage C4 and up to 0.45 mg ind−1 for adult females whereas lipids in C. finmarchicus represent only 0.02 mg ind−1 for copepodite stage C4 and 0.08 mg ind−1 for adult females (Scott et al. 2000).
Large adults caught in Rijpfjorden revealed higher proportion of total lipid compared to the adults caught in Kongsfjorden at the same period of the year. Difference between the two fjords was most probably link to the age of the population. In Rijpfjorden, organism length ranged from 22 to 40 mm. As discussed previously adults might reach 30 mm at the end of their first winter which suggests that in Rijpfjorden most of the organisms were in their second year generation whereas only first year generation individuals were found in Kongsfjorden. Thus adults might continue accumulating lipids during the second productive season of their life cycle which most probably might improve their reproduction and survival capacities.
Lipid storage of Themisto libellula and underlying mechanisms
Lipid class composition of juveniles showed TAG levels close to 20% TL during both years whereas WE accounted for 25% TL in 2006 and only 8% in 2007. For juveniles, intermediate levels were found by Scott et al. (1999) with 10.5% of TAG and 17.7% of WE. But they also recorded high quantity of FFA (10–15% TL) which indicates lipid degradation and hence make the comparison between these studies difficult. For large adults, Auel et al. (2002) described WE as the major lipid class with 42.6% TL while we observed for organisms of the same length in Rijpfjorden, lower proportion in 2006 (34% TL) and similar proportion in 2007 (40.2% TL). In addition, TAG proportions were slightly higher in our study (43.6 and 35.5% TL in 2006 and 2007 respectively) than in Auel et al. (2002) study (29.8% TL). Relatively high level of FFA was also measured in their samples making the comparison delicate. However these studies showed that T. libellula is able to store both lipid classes in different proportions, depending most probably on developmental stages considered and their environmental conditions. This contrasts with the Antarctic congener species Themisto gaudichaudii which accumulated TAG in large extent (more than 60% TL) and only low level or traces of WE (5.6% TL, Mayzaud et al. (1998) and <1% TL, Nelson et al. (2001) and Phleger et al. (1998)).
In Kongsfjorden, the first year organisms stored an intermediate and almost constant pool of fatty acids as TAG throughout the studied period (never more than 25% TL) while the TAG level of second year adults caught in Rijpfjorden, accounted for more than 40% TL. TAG are usually accumulated by organisms which are able to feed throughout the year and are thus considered as a short term lipid storage to fuel energetic demand during short period of low food availability (e.g. Meganyctiphanes norvegica, Falk-Petersen et al. 1981; Apherusa glacialis, Scott et al. 1999; Euphausia superba, Hagen et al. 2001; Themisto gaudichaudii, Nelson et al. 2001). Although no data exist on the winter feeding behaviour of T. libellula, it is most likely that they should be able to find food the whole year confirming the role of TAG to face up short starvation periods. Results from starvation experiments suggested also that despite a high potential to survive without food (about 168 days), T. libellula would have to feed during winter to fuel their energetic needs (Percy 1993a).
Proportions of WE, the other dominant lipid class of T. libellula, increased along growth during both years in Kongsfjorden. In 2006, WE level of adults exceeded TAG level and were even accumulated (i.e. in disproportion of their weight) whereas in the particular year 2007, adults had similar levels of WE and TAG. WE is considered as a long term storage lipid and according to Lee et al. (2006), WE would be more energetically valuable than TAG as they are more carefully regulated. It is generally accepted that carnivorous and omnivorous species accumulate less WE than do herbivorous species because there are less dependent on punctual blooms as they should be able to find food the whole year (Falk-Petersen et al. 1999; Lee et al. 2006). The studies on calanoid copepods revealed also that formation of WE may be in part a mechanism to remove end product inhibition of de novo fatty acid synthetase, enables thus a rapid lipogenesis despite a continuous input of dietary fatty acids as in bloom conditions (Sargent and Henderson 1986). Although the reason why and how T. libellula accumulated more WE during its first year generation are still unknown, it is noteworthy that other non-herbivorous species as Thysanoessa macrura or T. inermis (Falk-Petersen 1981; Hagen and Kattner 1998) stored also WE in relatively high level. The underlying hypothesis is that WE are partly involved in reproduction processes as this one occurred in winter when the food availability is limited. Hence these species, including T. libellula, need internal reserves which could be WE to fuel reproduction. Winter data are definitively needed to conclude on the role of each lipid classes however it seems that both TAG and WE play a role in T. libellula life strategy, in various extent depending on their physiological needs and environmental conditions.
References
Ackman RG (1981) Application of flame ionization detectors to thin layer chromatography on coated quartz rods. Methods Enzym 72:205–252
Auel H, Harjes M, da Rocha R, Stübing D, Hagen W (2002) Lipid biomarkers indicate different ecological niches and trophic relationships of the Arctic hyperiid amphipods Themisto abyssorum and T. libellula. Polar Biol 25:374–383
Basedow SL, Eiane K, Tverberg V, Spindler M (2004) Advection of zooplankton in an Arctic fjord (Kongsfjorden, Svalbard). Estuar Coast Shelf Sci 60:113–124
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bowman TE (1960) The pelagic amphipod genus Parathemisto (Hyperiidea: Hyperiidae) in the North Pacific and adjacent Arctic Ocean. Proc U.S. Natl Mus 112:343–392
Dale K, Falk-Petersen S, Hop H, Fevolden SE (2006) Population dynamics and body composition of the Arctic hyperiid amphipod Themisto libellula in Svalbard fjords. Polar Biol 29:1063–1070
Dalpadado P (2002) Inter-specific variations in distribution, abundance and possible life-cycle patterns of Themisto spp. (Amphipoda) in the Barents Sea. Polar Biol 25:656–666
Dalpadado P, Bogstad B (2004) Diet of juvenile cod (age 0–2) in the Barents Sea in relation to food availability and cod growth. Polar Biol 27:140–154
Dalpadado P, Borkner N, Bogstad B, Mehl S (2001) Distribution of Themisto spp. (Amphipoda) in the Barents Sea and predator-prey interactions. ICES J Mar Sci 58:876–895
Dalpadado P, Yamaguchi A, Ellertsen B, Johannessen S (2008) Trophic interactions of macro-zooplankton (krill and amphipods) in the Marginal Ice Zone of the Barents Sea. Deep Sea Res Part II 55:2266–2274
Dunbar MJ (1946) On Themisto libellula in Baffin Island Coastal waters. J Fish Res Board Can 6:419–434
Dunbar MJ (1957) The determinants of production in the northern seas: a study of the biology of Themisto libellula (Mandt). Can J Zool 35:797–819
Falk-Petersen S (1981) Ecological investigations on the zooplankton community in Balsfjorden, northern Norway: seasonal changes in body weight and the main biochemical composition of Thysanoessa inermis (Krøyer), T. raschii (M. Sars), and Meganictyphanes norvegica (M. Sars) in relation to environmental factors. J Exp Mar Biol Ecol 49:103–120
Falk-Petersen S, Gatten RR, Sargent JR, Hopkins CCE (1981) Ecological investigations on the zooplankton community in Balsfjorden, northern Norway: seasonal changes in the lipid class composition of Meganyctiphanes norvegica (M. Sars), Thysanoessa raschii (M. Sars), and T. inermis (Krøyer). J Exp Mar Biol Ecol 54:209–224
Falk-Petersen S, Hopkins CCE, Sargent JR (1990) Trophic relationships in the pelagic Arctic food web. In: Barnes M, Gibson RN (eds) Trophic relationships in the marine environment. Proceedings of the 24th European Marine Biology Symposium. Aberdeen University Press, Aberdeen, pp 315–333
Falk-Petersen S, Sargent JR, Lønne OJ, Timofeev S (1999) Functional biodiversity of lipids in Antarctic zooplankton: Calanoides acustus, Calanus propinquus, Thysanoessa macrura and Euphausia crystallorophias. Polar Biol 21:37–47
Falk-Petersen S, Haug T, Nilssen KT, Wold A, Dahl TM (2004) Lipids and trophic linkages in harp seal (Phoca groenlandica) from the eastern Barents Sea. Polar Res 23:43–50
Falk-Petersen S, Pavlov V, Timofeev S, Sargent JR (2007) Climate variability and possible effects on Arctic food chains: the role of Calanus. In: Ørbæk JB, Kallenborn R, Tombre I, Hegseth EN, Falk-Petersen S, Hoel AH (eds) Arctic Alpine ecosystems and people in a changing environment. Springer Verlag, Berlin, pp 147–166
Hagen W, Kattner G (1998) Lipid metabolism of Antarctic euphausiid Thysanoessa macrura and its ecological implications. Limnol Oceanogr 43:1894–1901
Hagen W, Kattner G, Terbrüggen A, Van Vleet ES (2001) Lipid metabolism of the Antarctic krill Euphausia superba and its ecological implications. Marine Biol 139:95–104
Haug T, Nilssen KT, Lindblom L (2004) Feeding habits of harp and hooded seals in drift ice waters along the east coast of Greenland in summer and winter. Polar Res 23:35–42
Hop H, Welch HE, Crawford RE (1997) Population structure and feeding ecology of Arctic cod (Boreogadus saida) in the Canadian High Arctic. In: Reynolds J (ed) Fish ecology in Arctic North America. American Fisheries Society Symposium 19. American Fisheries Society, Bethesda, pp 66–80
Kattner G, Hagen W (2008) Lipids in marine copepods: latitudinal characteristics and perspecive to global warming. In: Arts MT, Brett M, Kainz M (eds) Lipids in aquatic ecosystems (in press)
Kattner G, Krause M (1987) Changes in lipids during the development of Calanus finmarchicus s.l. from copepodid I to adult. Mar Biol 96:511–518
Kattner G, Graeve M, Hagen W (1994) Ontogenetic and seasonal changes in lipid and fatty acid/alcohol compositions of the dominant Antarctic copepods Calanus propinquus, Calanoides acutus and Rhincalanus gigas. Mar Biol 118:637–644
Koszteyn J, Timofeev S, Weslawski JM, Malinga B (1995) Size structure of Themisto abyssorum (Boeck) and Themisto libellula (Mandt) populations in European Arctic seas. Polar Biol 15:85–92
Labat J-P, Mayzaud P, Sabini S (2005) Population dynamics of Themisto gaudichaudii in Kerguelen Islands waters, Southern Indian Ocean. Polar Biol 28:776–783
Lee RF, Hagen W, Kattner G (2006) Lipid storage in marine zooplankton. Mar Ecol Prog Ser 307:273–306
Lønne OJ, Gabrielsen GW (1992) Summer diet of seabirds feeding in sea-ice-covered waters near Svalbard. Polar Biol 12:685–692
Marion A, Harvey M, Chabot D, Brêthes J-C (2008) Feeding ecology and predation impact of the recently established amphipod, Themisto libellula, in the St. Lawrence marine system, Canada. Mar Ecol Prog Ser 373:53–70
Mayzaud P, Errhif A, Bedo A (1998) Distribution of plankton lipids and their role in the biological transformation of Antarctic primary production. J Mar Syst 17:391–410
Nelson MM, Mooney BD, Nichols PD, Phleger CF (2001) Lipids of Antarctic Ocean amphipods: food chain interactions and the occurrence of novel biomarkers. Mar Chem 73:53–64
Nilssen KT, Haug T, Potelov V, Timoshenko YK (1995) Feeding habits of harp seals (Phoca groenlandica) during early summer and autumn in the northern Barents Sea. Polar Biol 15:485–493
Noyon M (2009) Nutrition and lipid dynamic of the hyperiid Themisto libellula. Importance in terms of adaptive strategy. Dr. philos. thesis, Paris, France, pp 139
Noyon M, Gasparini S, Mayzaud P (2009) Feeding of Themisto libellula (Amphipoda Crustacea) on natural copepods assemblages in an Arctic fjord (Kongsfjorden, Svalbard). Polar Biol 32:1559–1570
Percy JA (1993a) Energy consumption and metabolism during starvation in the Arctic hyperiid amphipod Themisto libellula Mandt. Polar Biol 13:549–555
Percy JA (1993b) Reproduction and growth of the Arctic hyperiid amphipod Themisto libellula (Mandt). Polar Biol 13:131–139
Phleger CF, Nichols PD, Virtue P (1998) Lipids and trophodynamics of Antarctic zooplankton. Comp Biochem Physiol B Comp Biochem 120:311–323
Sargent JR, Henderson RJ (1986) Lipids. In: Corner EDS, O’Hara SCM (eds) The biological chemistry of marine copepods. Clarendon, Oxford, pp 59–108
Scott CL, Falk-Petersen S, Sargent JR, Hop H, Lønne OJ, Poltermann M (1999) Lipids and trophic interactions of ice fauna and pelagic zooplankton in the marginal ice zone of the Barents Sea. Polar Biol 21:65–70
Scott CL, Kwasniewski S, Falk-Petersen S, Sargent JR (2000) Lipids and life strategies of Calanus finmarchicus, Calanus glacialis and Calanus hyperboreus in late autumn, Kongsfjorden, Svalbard. Polar Biol 23:510–516
Stempniewicz L, Blachowiak-Samolyk K, Weslawski JM (2007) Impact of climate change on zooplankton communities, seabird populations and arctic terrestrial ecosystem–A scenario. Deep Sea Res Part II 54:2934–2945
Willis KJ, Cottier FR, Kwasniewski S, Wold A, Falk-Petersen S (2006) The influence of advection on zooplankton community composition in an Arctic fjord (Kongsfjorden, Svalbard). J Mar Syst 61:39–54
Willis KJ, Cottier FR, Kwasniewski S (2008) Impact of warm advection on the winter zooplankton community in an Arctic fjord. Polar Biol 31:475–481
Wing BL (1976) Ecology of Parathemisto libellula and P. pacifica (Amphipoda: Hyperiidae) in Alaskan coastal waters. Northwest Fish Cent Proc Rep, March 1976. U.S. Nat Mar Fish Serv, pp 252–266
Acknowledgments
Thanks to Stig Falk-Peterson and Haakon Hop who allowed me to join cruises, the crew of the R/V Jan Mayen, R/V Lance and Kings Bay for sampling. Marc Boutoute for his help on lipid analyzes. We are also grateful to the referee for their helpful comments on the manuscript. This work is a contribution to two projects: Praceal project 455 funded by the French Polar Institute Paul Emile Victor (IPEV) and MariClim project 165112/S30 funded by the Research Council of Norway.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by X. Irigoien.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Noyon, M., Narcy, F., Gasparini, S. et al. Growth and lipid class composition of the Arctic pelagic amphipod Themisto libellula . Mar Biol 158, 883–892 (2011). https://doi.org/10.1007/s00227-010-1615-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-010-1615-1