Abstract
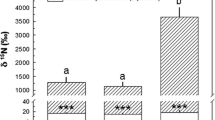
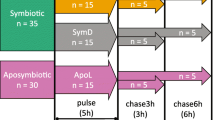
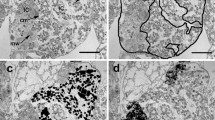
The relationship between anemones and anemonefishes is an oft-cited and endearing example of a mutualistic symbiosis. Current research on mutualistic symbioses suggests these relationships are more commonplace and have greater importance at the ecosystem level on nutrient dynamics and evolutionary processes than previously thought. Using stable isotopes 15N and 13C, both field and laboratory experiments were designed to investigate whether nutrient transfer from two species of resident anemonefishes (Amphiprion perideraion and A. clarkii) to host anemones (Heteractis crispa) occurs. Mass spectroscopy indicated that both 15N and 13C were significantly elevated in the tissues of anemonefishes and in both host anemone and zooxanthellae fractions. These experiments provide the first direct empirical evidence of nitrogen and carbon transfer from resident anemonefishes to host anemones and endosymbiotic zooxanthellae. Such transfer of elements within this intriguing tripartite association underscores the central role that nutrient dynamics contributes to the evolutionary processes of these marine symbioses.






Similar content being viewed by others
References
Arnal C, Côté IM, Morand S (2001) Why clean and be cleaned? The importance of client ectoparasites and mucus in a marine cleaning symbiosis. Behav Ecol Sociobiol 51:1–7. doi:10.1007/s002650100407
Arvedlund M, Iwao K, Brolund TM, Takemura A (2006) Juvenile Thalassoma amblycephalum Bleeker (Labridae, Teleostei) dwelling among the tentacles of sea anemones: a cleanerfish with an ususual client? J Exp Mar Biol Ecol 329:161–173. doi:10.1016/j.jembe.2005.08.005
Bachar A, Achituv Y, Pasternak Z, Dubinsky Z (2007) Autotrophy versus heterotrophy: the origin of carbon determines its fate in a symbiotic sea anemone. J Exp Mar Biol Ecol 349:295–298. doi:10.1016/j.jembe.2007.05.030
Belda CA, Lucas JS, Yellowlees D (1993) Nutrient limitation in the giant clam-zooxanthellae symbiosis: effects of nutrient supplements on growth of the symbiotic partners. Mar Biol 117:655–664. doi:10.1007/BF00349778
Biel KY, Gates RD, Muscatine L (2007) Effects of free amino acids on the photosynthetic carbon metabolism of symbiotic dinoflagellates. Russian J Plant Physiol 54:171–183. doi:10.1134/S1021443707020033
Brooks WR, Mariscal RN (1984) The acclimation of anemone fishes to sea anemones: protection by changes in the fish’s mucous coat. J Exp Mar Biol Ecol 80:277–285. doi:10.1016/0022-0981(84)90155-2
Buston PM (2003) Mortality is associated with social rank in the clown anemonefish (Amphiprion percula). Mar Biol 143:811–815. doi:10.1007/s00227-003-1106-8
Cleveland A, Montgomery WL (2003) Gut characteristics and assimilation efficiencies in two species of herbivorous damselfishes (Pomacentridae: Stegastes dorsopunicans and S. planifrons). Mar Biol 142:35–44. doi:10.1007/s00227-002-0916-4
Cleveland A, Jackson HR, Verde EA (2006) Behavioral comparisons of two sympatric clownfish species (Amphiprion clarkia and A. perideraion) as symbionts of the anemone Heteractis crispa. Ecol Evol Ethol Fish Symp, Soka University, Aliso Viejo
Cocheret de la Morinière E, Pollux BJA, Nagelkerken I, Hemminga MA, Huiskes AHL, van der Velde G (2003) Ontogenetic dietary changes of coral reef fishes in the mangrove-seagrass-reef continuum: stable isotopes and gut-content analysis. Mar Ecol Prog Ser 246:279–289. doi:10.3354/meps246279
D’Elia CF, Webb KL (1977) The dissolved nitrogen flux of reef corals. In: Proceedings of 3rd International Coral Reef Symposium, Miami
D’Elia CF, Domoster SL, Webb KL (1983) Nutrient uptake kinetics of freshly isolated zooxanthellae. Mar Biol 75:157–167. doi:10.1007/BF00405998
Davies PS (1992) Endosymbiosis in marine cnidarians. In: John DM, Hawkins SJ, Prince JH (eds) Plant-animal interactions in the marine benthos. Clarendon Press, Oxford, pp 511–540
Davy SK, Trautman DA, Borowitzka MA, Hinde R (2002) Ammonium excretion by a symbiotic sponge supplies the nitrogen requirements of its rhodophyte partner. J Exp Biol 205:3505–3511
DeFreese DE, Clark KB (1991) Transepidermal uptake of dissolved free amino acids from seawater by three ascoglossan opisthobranchs. J Molluscan Stud 57:65–74. doi:10.1093/mollus/57.Supplement_Part_4.65
Depczynski M, Fulton CJ, Marnane MJ, Bellwood DR (2007) Life history patterns shape energy allocation among fishes on coral reefs. Oecologia 153:111–120. doi:10.1007/s00442-007-0714-2
Fautin DG (1991) The anemonefish symbiosis: what is known and what is not. Symbiosis 10:23–46
Fautin DG, Allen GR (1992) Field guide to anemonefishes and their host sea anemones. Western Australia Museum, Perth
Fautin DG, Guo C-C, Hwang J-S (1995) Costs and benefits of the symbiosis between the anemoneshrimp Periclimenes brevicarpalis and its host Entacmaea quadricolor. Mar Ecol Prog Ser 129:77–84. doi:10.3354/meps129077
Ferguson JC (1982) A comparative study of the net metabolic benefits derived from the uptake and release of free amino acids by marine invertebrates. Biol Bull 162:1–17
Fitt WK, Cook CB (2001) The effects of feeding or addition of dissolved inorganic nutrients in maintaining the symbiosis between dinoflagellates and a tropical marine cnidarians. Mar Biol 139:507–517. doi:10.1007/s002270100598
Fricke HW (1979) Mating system, resource defense and sex change in the anemonefish, Amphiprion akallopisos. Z Tierpsychol 50:313–326
Furla P, Allemand D, Schick JM, Ferrier-Pages C, Richier S, Plantivaux A, Merle P, Tambutte S (2005) The symbiotic anthozoan: a physiological chimera between alga and animal. Integr Comp Biol 45:595–604. doi:10.1093/icb/45.4.595
Gerking SD (1994) Feeding ecology of fish. Academic Press, San Diego
Godinot C, Chadwick NE (2009) Phosphate excretion by anemonefish and uptake by giant sea anemones: demand outstrips supply. Bull Mar Sci 85:1–9
Godwin J, Fautin DG (1992) Defense of host actinians by anemonefishes. Copeia 1992(3):902–908
Goldshmid R, Holzman R, Weihs D, Genin A (2004) Aeration of corals by sleep-swimming fish. Limnol Oceanogr 49:1832–1839
Gomme J (1982) Epidermal nutrient absorption in marine invertebrates: a comparative analysis. Amer Zool 22:691–708. doi:10.1093/icb/22.3.691
Hattori A (2002) Small and large anemonefishes can coexist using the same patchy resources on a coral reef, before habitat destruction. J Anim Ecol 71:824–831. doi:10.1046/j.1365-2656.2002.00649.x
Heikoop JM, Risk MJ, Lazier AV, Edinger EN, Jompa J, Limmon GV, Dunn JJ, Browne DR, Schwarcz HP (2000) Nitrogen-15 signals of anthropogenic nutrient loading in reef corals. Mar Poll Bull 40:628–636. doi:10.1016/S0025-326X(00)00006-0
Ho C-T, Kao S-J, Dai C-F, Hsieh H-L, Shiah F-K, Jan K-Q (2007) Dietary separation between two blennies and the Pacific Gregory in northern Taiwan: evidence from stomach content and stable isotope analysis. Mar Biol 151:729–736. doi:10.1007/s00227-006-0517-8
Hoegh-Guldberg O, Smith GJ (1989) The effect of sudden changes in temperature, light and salinity on the population density and export of zooxanthellae from the reef corals Stylophora pistillata Esper and Seriatopora hystrix Dana. J Exp Mar Biol Ecol 129:279–303. doi:10.1016/0022-0981(89)90109-3
Holbrook SJ, Schmitt RJ (2005) Growth, reproduction and survival of a tropical sea anemone (Actinaria): benefits of hosting anemonefish. Coral Reefs 24:67–73. doi:10.1007/s00338-004-0432-8
Holbrook SJ, Brooks AJ, Schmitt RJ, Stewart HL (2008) Effects of sheltering fish on growth of their coral hosts. Mar Biol 155:521–530. doi:10.1007/s00227-008-1051-7
Houlbrèque F, Ferrier-Pagès C (2009) Heterotrophy in tropical scleractinian corals. Biol Rev 84:1–17. doi:10.1111/j.1469-185X.2008.00058.x
Klumpp DW, Polunin NVC (1989) Partitioning among grazers of food resources within damselfish territories on a coral reef. J Exp Mar Biol Ecol 125:145–169. doi:10.1016/0022-0981(89)90040-3
Liberman T, Genin A, Loya Y (1995) Effects on growth and reproduction of the coral Stylophora pistillata by the mutualistic damselfish Dascyllus marginatus. Mar Biol 121:741–746. doi:10.1007/BF00349310
Lipschultz F, Cook CB (2002) Uptake and assimilation of 15N-ammonium by the symbiotic sea anemone Batholomea annulata and Aiptasia pallida: conservation versus recycling of nitrogen. Mar Biol 140:489–502. doi:10.1007/s00227-001-0717-1
Marshal WS, Grosell M (2006) Ion transport, osmoregulation, and acid-base regulation. In: Evans DH, Claiborne JB (eds) The physiology of fishes, 3rd edn. CRC Press, Boca Raton, pp 177–230
Meyer JL, Schultz ET (1985) Tissue condition and growth rate of corals associated with schooling fish. Limnol Oceanogr 30:157–166
Meyer JL, Schultz ET, Helfman GS (1983) Fish schools: an asset to corals. Science 220:1047–1049. doi:10.1126/science.220.4601.1047
Montgomery WL, Gerking SD (1980) Marine macroalgae as foods for fishes: an evaluation of potential food quality. Environ Biol Fish 5:143–153. doi:10.1007/BF02391621
Muscatine L (1990) The role of symbiotic algae in carbon and energy flux in reef corals. In: Dubinsky Z (ed) Coral reefs. Elsevier, Amsterdam
Muscatine L, D’Elia CF (1978) The uptake, retention, and release of ammonium by reef corals. Limnol Oceanogr 23:725–734
Muscatine L, Kaplan IR (1994) Resource partitioning by reef corals as determined from stable isotope composition II. 15N of zooxanthellae and animal tissue versus depth. Pac Sci 48:304–312
Muscatine L, Porter JW (1977) Reef corals and mutualistic symbiosis adapted to nutrient-poor environment. BioSci 27:454–460
Nakagawa H, Asakawa M, Enomoto N (1988) Diversity in the carbohydrate moieties of mucus glycoproteins of various fishes. Nippon Suisan Gakkaishi 54:1653–1658
Pinnegar JK, Polunin NVC (2006) Planktivorous damselfish supports significant nitrogen and phosphorus fluxes to Mediterranean reefs. Mar Biol 148:1089–1099. doi:10.1007/s00227-005-0141-z
Polunin NVC (1988) Efficient uptake of algal production by a single resident herbivorous fish on a reef. J Exp Mar Biol Ecol 123:61–76. doi:10.1126/science.220.4601.1047
Porat D, Chadwick-Furman NE (2004) Effects of anemonefish on giant sea anemones: expansion behavior, growth, and survival. Hydrobiologica 530–531:513–520. doi:10.1007/978-1-4020-2762-8_58
Porat D, Chadwick-Furman NE (2005) Effects of anemonefish on giant sea anemones: ammonium uptake, zooxanthella content and tissue regeneration. Mar Fresh Behav Physiol 38:43–51. doi:10.1080/10236240500057929
Richardson DL, Harrison PL, Harriott VJ (1997) Timing of spawning and fecundity of a tropical and subtropical anemonefish (Pomacentridae: Amphiprion) on a high latitude reef on the east coast of Australia. Mar Ecol Prog Ser 156:175–181
Rinkevich B, Wolodarsky Z, Loya Y (1991) Coral-crab association: a compact domain of a multilevel trophic system. Hydrobiologia 216/217:279–284. doi:10.1007/BF00026475
Roberts JM, Davies PS, Fixter LM, Preston T (1999) Primary site and initial products of ammonium assimilation in the symbiotic sea anemone Anemonia viridis. Mar Biol 135:223–236. doi:10.1007/s002270050620
Roberts CM, McClean CJ, Veron JEN, Hawkins JP, Allen GR, McAllister DE, Mittermeier CG, Schueler FW, Spalding M, Wells F, Vynne C, Werner TB (2002) Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295:1280–1284. doi:10.1126/science.1067728
Roopin M, Chadwick NE (2009) Benefits to host sea anemones from ammonia contributions of resident anemonefishes. J Exp Mar Biol Ecol 370:27–34. doi:10.1016/j.jembe.2008.11.006
Roopin M, Henry RP, Chadwick NE (2008) Nutrient transfer in a marine mutualism: patterns of ammonia excretion by anemonefish and uptake by giant sea anemones. Mar Biol 154:547–556. doi:10.1007/s00227-008-0948-5
Schmitt RJ, Holbrook SJ (2003) Mutualism can mediate competition and promote coexistence. Ecol Letters 6:898–902. doi:10.1046/j.1461-0248.2003.00514.x
Shuman CS, Hodgson G, Ambrose RF (2005) Population impacts of collecting sea anemones and anemonefish for the marine aquarium trade in the Philippines. Coral Reefs 24:564–573. doi:10.1007/s00338-005-0027-z
Stewart HL, Holbrook SJ, Schmitt RJ, Brooks AJ (2006) Symbiotic crabs maintain coral health by clearing sediments. Coral Reefs 25:609–615. doi:10.1007/s00338-006-0132-7
Tanaka Y, Miyajima T, Koike I, Hayashibara T, Ogawa H (2006) Translocation and conservation of organic nitrogen within the coral-zooxanthella symbiotic system of Acropora pulchra, as demonstrated by dual isotope-labeling techniques. J Exp Mar Biol Ecol 336:110–119. doi:10.1016/j.jembe.2006.04.011
Tyler WA, Stanton F (1995) Potential influence of food abundance on spawning patterns in a damselfish, Abudefduf abdominalis. Bull Mar Sci 57:610–623
Venn AA, Loram JE, Douglas AE (2008) Photosynthetic symbioses in animals. J Exper Bot 59:1069–1080. doi:10.1093/jxb/erm328
Walsh PJ, Blackwelder P, Gill KA, Danulat E, Mommsen TP (1991) Carbonate deposits in marine fish intestines: a new source of biomineralization. Limnol Oceangr 36:1227–1232
Wang J-T, Douglas AE (1998) Nitrogen recycling or nitrogen conservation in an alga-invertebrate symbiosis? J Exp Mar Biol Ecol 201:2445–2453
Wang J-T, Douglas AE (1999) Essential amino acid synthesis and nitrogen recycling for an alga-invertebrate symbiosis. Mar Biol 135:219–222. doi:10.1007/s002270050619
Wilkerson FP, Trench RK (1986) Uptake of dissolved inorganic nitrogen by the symbiotic clam Tridacna gigas and the coral Acropora sp. Mar Biol 93:237–246. doi:10.1007/BF00508261
Wilson RW, Millero FJ, Taylor JR, Walsh PJ, Christensen V, Jennings S, Grosell M (2009) Contribution of fish to the marine inorganic carbon cycle. Science 323:359–362. doi:10.1126/science.1157972
Yellowlees D, Rees RAV, Leggat W (2008) Metabolic interactions between algal symbionts and invertebrate hosts. Plant Cell Environ 31:679–694. doi:10.1111/j.1365-3040.2008.01802.x
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice-Hall, New Jersey
Acknowledgments
We would like to thank D. Forest, H. Jackson, C. Blair, and D. Dallis for field and laboratory assistance. We also thank H. Calumpong and the researchers and staff at Silliman University, Dumaguete, Philippines, for laboratory and logistical assistance. Financial support was provided by Sigma Delta Epsilon Graduate Women in Science (AC), the American Philosophical Society (AC), PADI A.W.A.R.E. (AC, EAV), and Maine Maritime Academy Professional Development Committee (AC, EAV). We thank our three anonymous reviewers whose constructive comments greatly enhanced the merit of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Goulet.
Rights and permissions
About this article
Cite this article
Cleveland, A., Verde, E.A. & Lee, R.W. Nutritional exchange in a tropical tripartite symbiosis: direct evidence for the transfer of nutrients from anemonefish to host anemone and zooxanthellae. Mar Biol 158, 589–602 (2011). https://doi.org/10.1007/s00227-010-1583-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-010-1583-5