Abstract
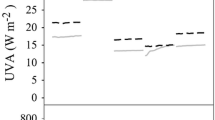
Large quantities of floating macroalgae are traveling in coastal waters of the SE Pacific and in other temperate climate zones. While afloat, these algae are potentially exposed to full solar radiation, including UVA and UVB, which can have profound effects on their physiological and growth performance. Latitudinal variations in UV-radiation (UVR) are hypothesized to affect floating algae differently with higher impacts at low latitudes than at high latitudes. In addition, UVR together with grazing might accelerate the demise of floating kelps. This hypothesis was tested with outdoor laboratory experiments in which sporophytes of the giant kelp Macrocystis pyrifera (L.) C. Agardh were exposed to a combination of different UVR regimes (PAR only, PAR + UV) and grazing at three sites along the Chilean coast (20°S, 30°S, and 40°S). A latitudinal trend in irradiance was detected with increasing values from 40°S to 20°S. Surprisingly, floating M. pyrifera responded with a high acclimation potential within this latitudinal UVR gradient. At 20°S, floating kelps were slightly sensitive to UVR, which was reflected in reduced blade growth. At 30°S, physiological responses were hardly affected by the prevailing irradiance but sporophyte growth and thus persistence mainly depended on the presence or absence of amphipod grazers. At high latitudes, grazing had only minor impacts on algal biomass and blade growth, and kelps thrived well under all tested environmental conditions. Overall, our results reveal that floating M. pyrifera was only slightly affected by UVR and that sporophytes can efficiently acclimate over a latitudinal UVR gradient that spans from 20°S to 40°S. Given this high acclimation potential, we suggest that these (and possibly other) positively buoyant algae are important dispersal agents over a wide range of temperate latitude conditions.





Similar content being viewed by others
References
Aguilera J, Karsten U, Lippert H, Vögele B, Philipp E, Hanelt D, Wiencke C (1999) Effects of solar radiation on growth, photosynthesis and respiration of marine macroalgae from the Arctic. Mar Ecol Prog Ser 191:109–119
Aguirre-von-Wobeser E, Figueroa FL, Cabello-Pasini A (2000) Effect of UV radiation on photoinhibition of marine macrophytes in culture systems. J Appl Phycol 12:159–168
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46
Anderson MJ (2005) PERMANOVA: a FORTAN computer program for permutational multivariate analysis of variance. Department of Statistics, University of Auckland, New Zealand
Bischof K, Hanelt D, Tüg H, Karsten U, Brouwer PEM, Wiencke C (1998) Acclimation of brown algal photosynthesis to ultraviolet radiation in Arctic coastal waters (Spitsbergen, Norway). Polar Biol 20:388–395
Bischof K, Hanelt D, Aguilera J, Karsten U, Vögele B, Sawall T, Wiencke C (2002) Seasonal variation in ecophysiological patterns in macroalgae from an Arctic fjord. I. Sensitivity of photosynthesis to ultraviolet radiation. Mar Biol 140:1097–1106
Bischof K, Gómez I, Molis M, Hanelt D, Karsten U, Lüder U, Roleda MY, Zacher K, Wiencke C (2006) Ultraviolet radiation shapes seaweed communities. Rev Environ Sci Biotechnol 5:141–166
Broitman BR, Navarrete SA, Smith F, Gaines SD (2001) Geographic variation of southeastern Pacific intertidal communities. Mar Ecol Prog Ser 224:21–34
Buschmann AH, Vásquez JA, Osorio P, Reyes E, Filón L, Hernández-González MC, Vega A (2004) The effect of water movement, temperature and salinity on abundance and reproductive patterns of Macrocystis spp. (Phaeophyta) at different latitudes in Chile. Mar Biol 145:849–862
Cabello-Pasini A, Aguirre-von-Wobeser E, Figueroa FL (2000) Photoinhibition of photosynthesis in Macrocystis pyrifera (Phaeophyceae), Chondrus crispus (Rhodophyceae) and Ulva lactuca (Chlorophyceae) in outdoor culture systems. J Photochem Photobiol B Biol 57:169–178
Cerda O, Karsten U, Rothäusler E, Tala F, Thiel M (2009) Compensatory growth of the kelp Macrocystis integrifolia (Phaeophyceae, Laminariales) against grazing of Peramphithoe femorata (Amphipoda, Ampithoidae) in northern-central Chile. J Exp Mar Biol Ecol 377:61–67
Clendennen SK, Zimmerman RC, Powers DA, Aberte RS (1996) Photosynthetic response of the giant kelp Macrocystis pyrifera (Phaeophyceae) to ultraviolet radiation. J Phycol 32:614–620
Colombo-Pallotta MF, García-Mendoza E, Ladah LB (2006) Photosynthetic performance, light absorption, and pigment composition of Macrocystis pyrifera (Laminariales, Phaeophyceae) blades from different depths. J Phycol 42:1225–1234
Dean TA (1985) The temporal and spatial distribution of underwatrer quantum irradiation in a Southern California kelp forest. Estuar Coast Shelf Sci 21:835–844
Demes KW, Graham MH, Suskiewicz TS (2009) Phenotypic plasticity reconciles incongruous molecular and morphological taxonomies: giant kelp Macrocystis (Laminariales, Phaeophyceae) is a monospecific genus. J Phycol 45:1266–1269
Deysher L, Norton TA (1982) Dispersal and colonization in Sargassum muticum (Yendo) Fensholt. J Exp Mar Biol Ecol 56:179–195
Dring MJ, Makarov V, Schodchina E, Lorenz M, Lüning K (1996) Influence of ultraviolet-radiation on chlorophyll fluorescence and growth in different life-history stages of three species of Laminaria (Phaeophyta). Mar Biol 126:183–191
Edwards MS, Kim KY (2010) Diurnal variations in relative photosynthetic performance in giant kelp Macrocystis pyrifera (Phaeophyceae, Laminariales) at different depths as estimated using PAM fluorometry. Aquat Bot 92:119–128
Falkowski PG, LaRoche J (1991) Acclimation to spectral irradiance in algae. J Phycol 27:8–14
Figueroa FL, Salles S, Aguilera J, Jimenez C, Mercado J, Viñegla B, Flores-Moya A, Altamirano M, Helbling EW (1997) Effects of solar radiation on photoinhibition and pigmentation in the red alga Porphyra leucosticta. Mar Ecol Prog Ser 151:81–90
Figueroa FL, Martinez B, Israel A, Neori A, Malta EJ, Ang P, Inken S, Marquardt R, Frenk S, Korbee N (2009) Acclimation of Red Sea macroalgae to solar radiation: photosynthesis and thallus absorptance. Aquat Biol 7:159–172
Franklin LA, Forster RM (1997) The changing irradiance environment: consequences for marine macrophyte physiology, productivity and ecology. Eur J Phycol 32:207–232
Gaines SD, Lubchenco J (1982) A unified approach to marine plant herbivore interactions II biogeographic patterns. Annu Rev Ecol Syst 13:111–138
García-Mendoza E, Colombo-Pallotta MF (2007) The giant kelp Macrocystis pyrifera presents a different nonphotochemical quenching control than higher plants. New Phytol 173:526–536
Gerard VA (1982) Growth and utilization of internal nitrogen reserves by the giant kelp Macrocystis pyrifera in a low-nitrogen environment. Mar Biol 66:27–35
Gómez I, Figueroa FL, Ulloa N, Morales V, Lovengreen C, Huovinen P, Hess S (2004) Photosynthesis in 18 intertidal macroalgae from Southern Chile. Mar Ecol Progr Ser 270:103–116
Grzymski J, Johnsen G, Sakshaug E (1997) The significance of intracellular self-shading on the biooptical properties of brown, red, and green macroalgae. J Phycol 33:408–414
Gutow L (2003) Local population persistence as a pre-condition for large-scale dispersal of Idotea metallica (Crustacea, Isopoda) on drifting habitat patches. Hydrobiologia 503:45–48
Hanelt D (1998) Capability of dynamic photoinhibition in Arctic macroalgae is related to their depth distribution. Mar Biol 131:361–369
Hanelt D, Melchersmann B, Wiencke C, Nultsch W (1997) Effects of high light stress on photosynthesis of polar macroalgae in relation to depth distribution. Mar Ecol Prog Ser 149:255–266
Henley WJ, Dunton KH (1995) A seasonal comparison of carbon, nitrogen, and pigment content in Laminaria solidungula and L. saccharina (Phaeophyta) in the Alaskan Arctic. J Phycol 31:325–331
Hernández-Carmona G, Hughes B, Graham M (2006) Reproductive longevity of drifting kelp Macrocystis pyrifera (Phaeophyceae) in Monterey Bay, USA. J Phycol 42:1199–1207
Hinojosa IA, Boltaña S, Lancelloti D, Macaya E, Ugalde P, Valdiva N, Vásquez N, NewmanWA ThielM (2006) Geographic distribution and description of four pelagic barnacles along the south east Pacific coast of Chile—a zoogeographical approximation. Rev Chil Hist Nat 79:13–27
Hinojosa I, González E, Ugalde P, Valdivia N, Macaya E, Thiel M (2007) Distribution and abundance of floating seaweeds and their associated peracarid fauna in the fjords and channels of the XI. Region, Chile. Cienc Tecnol Mar (Chile) 30:37–50
Hobday AJ (2000) Age of drifting Macrocystis pyrifera (L.) C. Agardh rafts in the Southern California Bight. J Exp Mar Biol Ecol 253:97–114
Honkanen T, Jormalainen V (2002) Within-alga integration and compensation: effects of simulated herbivory on growth and reproduction of the brown alga Fucus vesiculosus. Int J Plant Sci 163:815–823
Hoyer K, Karsten U, Wiencke C (2002) Induction of sunscreen compounds in Antarctic macroalgae by different radiation conditions. Mar Biol 41:619–627
Inskeep WP, Bloom PR (1985) Extinction coefficients of Chlorophyll a and b in N, N-dimetylformamide and 80% acetone. Plant Physiol 77:483–485
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Karsten U (2008) Defense strategies of algae and cyanobacteria against solar ultraviolet radiation. In: Amsler CD (ed) Algal chemical ecology. Springer, New York, pp 273–295
Karsten U, Bischof K, Wiencke C (2001) Photosynthetic performance of Arctic macroalgae after transplantation from deep to shallow waters. Oecologia 127:11–20
Lobban CS, Harrison PJ (1994) Light and photosynthesis. In: Lobban CS, Harrison PJ (eds) Seaweed ecology and physiology. Cambridge University Press, Cambridge, pp 123–162
Macaya EC, Zuccarello GC (2010) DNA barcoding and genetic divergence in the giant kelp Macrocystis (Laminariales). J Phycol 46:736–742
Macaya EC, Boltaña S, Hinojosa IA, Macchiavello JE, Valdivia NA, Vásquez N, Buschmann AH, Vásquez J, Vega JMA, Thiel M (2005) Presence of sporophylls in floating kelp rafts of Macrocystis spp. (Phaeophycea) along the Chilean Pacific coast. J Phycol 41:915–922
Madronich S, McKenzie RL, Björn LO, Caldwell MM (1998) Changes in biologically active ultraviolet radiation reaching the Earth’s surface. J Photochem Photobiol B Biol 46:5–19
McArdle BH, Anderson MJ (2001) Fitting multivariate models to community data: a comment on distance-based redundancy analysis. Ecology 82:290–297
Mercado JM, Jiménez C, Niell FX, Figueroa FL (1996) Comparison of methods for measuring light absorption by algae and their application to the estimation of the package effect. Sci Mar 60:39–45
Michler T, Aguilera J, Hanelt D, Bischof K, Wiencke C (2002) Long-term effects of ultraviolet radiation on growth and photosynthetic performance of polar and cold-temperate macroalgae. Mar Biol 140:1117–1127
Navarro NP, Mansilla A, Palacios M (2008) UVB effects on early developmental stages of commercially important macroalgae in southern Chile. J Appl Phycol 20:897–906
Norton TA (1977) The growth and development of Sargassum muticum (Yendo) Fensholt. J Exp Mar Biol Ecol 26:41–53
Nygard CA, Ekelund NGA (2006) Photosynthesis and UV-B tolerance of the marine alga Fucus vesiculosus at different sea water salinities. J Appl Phycol 18:461–467
Pansch C, Gómez I, Rothäusler E, Véliz K, Thiel M (2008) Species-specific defense strategies of vegetative versus reproductive blades of the Pacific kelps Lessonia nigrescens and Macrocystis integrifolia. Mar Biol 155:51–62
Pavia H, Toth GB (2000) Inducible chemical resistance to herbivory in the brown seaweed Ascophyllum nodosum. Ecology 81:3212–3225
Pavia H, Cervin G, Lindgren A, Aberg P (1997) Effects of UVB radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum. Mar Ecol Prog Ser 157:139–146
Roleda MY, Wiencke C, Hanelt D, Bischof K (2007) Sensitivity of the early life stages of macroalgae from the northern hemisphere to ultraviolet radiation. Photochem Photobiol 83:851–862
Rothäusler E, Thiel M (2006) Effect of detachment status on the palatability of two kelp species. J Appl Phycol 18:423–435
Rothäusler E, Gómez I, Hinojosa IA, Karsten U, Tala F, Thiel M (2009) Effect of temperature and grazing on growth and reproduction of floating Macrocystis spp. (Phaephyceae) along a latitudinal gradient. J Phycol 45:547–559
Rothäusler E, Gómez I, Hinojosa IA, Karsten U, Tala F, Thiel M (accepted) Physiological performance of floating giant kelp Macrocystis pyrifera (Phaeophyceae): Latitudinal variability in the effects of temperature and grazing. J Phycol
Schreiber U, Bilger W, Neubauer C (1994) Chlorophyll fluorescence as a non intrusive indicator for rapid assessment of in vivo photosynthesis. Ecol Stud 100:49–70
Swanson AK, Druehl LD (2002) Induction, exudation and the UV protective role of kelp phlorotannins. Aquat Bot 73:241–253
Tala F, Edding M (2005) Growth and tissue loss in blades of Lessonia nigrescens and Lessonia trabeculata (Laminariales, Phaeophyceae) in northern Chile. Aquat Bot 82:39–54
Tala F, Véliz K, Gómez I, Edding M (2007) Early life stages of the south Pacific kelps Lessonia nigrescens and Lessonia trabeculata (Laminariales, Phaeophyceae) show recovery capacity following exposure to UV radiation. Phycologia 46:467–470
Thiel M, Gutow L (2005a) The ecology of rafting in the marine environment I. The floating substrata. Oceanogr Mar Biol Annu Rev 42:181–264
Thiel M, Gutow L (2005b) The ecology of rafting in the marine environment II. The rafting organisms and community. Oceanogr Mar Biol Annu Rev 43:279–418
Underwood AJ (1997) Experiments in ecology. Their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge
Van Alstyne KL (1988) Herbivore grazing increases polyphenolic defenses in the intertidal brown alga Fucus distichus. Ecology 69:655–663
Van de Poll WH, Eggert A, Buma GJ, Breeman A (2001) Effects of UV-B induced DNA damage and photoinhibition on growth of temperate marine red macrophytes: habitat-related differences in UV-B tolerance. J Phycol 37:30–37
Vandendriessche S, Vincx M, Degraer S (2007a) Floating seaweed and the influences of temperature, grazing and clump size on raft longevity—A microcosm study. J Exp Mar Biol Ecol 343:64–71
Vandendriessche S, Messiaen M, O’Flynn S, Vincx M, Degraer S (2007b) Hiding and feeding in floating seaweed: floating seaweed clumps as possible refuges or feeding grounds for fishes. Est Coast Shelf Sci 71:691–703
Véliz K, Edding M, Tala F, Gómez I (2006) Effects of ultraviolet radiation on different life cycle stages of the south Pacific kelps, Lessonia nigrescens and Lessonia trabeculata (Laminariales, Phaeophyceae). Mar Biol 149:115124
Von Ende CN (1993) Repeated-measures analysis: Growth and other time-dependent measures. In: Schreiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Chapman and Hall, New York, pp 113–137
Whitehead RF, de Mora SJ, Demers S (2000) Enhanced UV radiation—a new problem for the marine environment. In: de Mora S, Demers S, Vernet M (eds) The effects of UV radiation in the marine environment. Cambridge University Press, Cambridge, pp 1–34
Wiencke C, Clayton MN, Gómez I, Iken K, Lüder UH, Amsler CD, Karsten U, Hanelt K, Bischof K, Dunton K (2006) Life strategy, ecophysiology and ecology of seaweeds in polar waters. Rev Environ Sci Biotechnol 6:95–126
Wiencke C, Lüder UH, Roleda MY (2007) Impact of ultraviolet radiation on physiology and development of zoospores of the brown alga Alaria esculenta from Spitsbergen. Physiol Plant 130:601–612
Yakovleva IM, Titlyanov EA (2001) Effect of high visible and UV irradiance on subtidal Chondrus crispus: stress, photoinhibition and protective mechanisms. Aquat Bot 71:47–61
Acknowledgments
Financial support for this research was provided by FONDECYT 1060127 to MT, FT and IG. The first author is grateful to the German Academic Exchange Service (DAAD) and the FAZIT-Stiftung for financial support during her PhD thesis. We thank Ivan Hinojosa, Leonardo Miranda, Osvaldo Cerda, and Mario Villegas for their valuable help in the field and during the experiments. Finally, we are grateful to two anonymous reviewers who helped to improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Kraufvelin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rothäusler, E., Gómez, I., Karsten, U. et al. UV-radiation versus grazing pressure: long-term floating of kelp rafts (Macrocystis pyrifera) is facilitated by efficient photoacclimation but undermined by grazing losses. Mar Biol 158, 127–141 (2011). https://doi.org/10.1007/s00227-010-1547-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-010-1547-9