Abstract
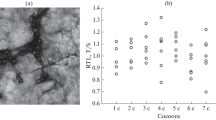
The polyp (scyphistoma) of the jellyfish Cassiopea andromeda reproduces asexually repeatedly, while the medusa, the sexually reproducing stage, exhibits a relatively shorter life span. As a first step to understand the mechanism behind the differences in the life spans of the polyp and medusa stages of the jellyfish, we compared the lengths of the telomere region of one targeted chromosome between the polyp and medusa stages using a modified single telomere length assay (STELA). The double-stranded regions of the telomeres were amplified by PCR, and the average length of the PCR products was estimated by densitometry analysis of the gel smear. Chromosomes within cells of the bell region of the medusa were characterized by longer telomeres than those of polyps, asexual propagules, or other regions of the medusa. This is the first study to estimate the telomere lengths of targeted chromosomes in a cnidarian and opens a way to understand the mechanism underlying different life spans of the polyp and medusa stages.





Similar content being viewed by others
References
Alverca E, Cuadrado A, Jouve N, Franca S, Moreno Diaz de la Espina S (2007) Telomeric DNA localization on dinoflagellate chromosomes: structural and evolutionary implications. Cytogenet Genome Res 116:224–231
Baird DM, Rowson J, Wynford-Thomas D, Kipling D (2003) Extensive allelic variation and ultrashort telomeres in senescent human cells. Nature Genet 33:203–207
Bigelow RP (1900) The anatomy and development of Cassiopea xamachana. Mem Boston Soc Nat Hist 5:191–236
Blackburn EH (1991) Structure and function of telomeres. Nature 350:569–573
Colley JN, Trench RK (1983) Selectivity in phagocytosis and persistence of symbiotic algae by the scyphistoma stage of the jellyfish Cassiopeia xamachana. Proc R Soc Lond B 219:61–82
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Gohar HAF, Eisawy AM (1960) The biology of Cassiopea andromeda (from the Red Sea) (With a note on the species problem). Publ Mar Biol Stat Ghardaqa 11:3–39
Greider CW (1990) Telomeres, telomerase and senescence. Bioessays 12:363–369
Harley CB (1991) Telomere loss: mitotic clock or genetic time bomb? Mutat Res 256:271–282
Hofmann DK, Honegger TG (1990) Bud formation and metamorphosis in Cassiopea andromeda (Cnidaria: Scyphozoa): a developmental and ultrastructural study. Mar Biol 105:509–518
Hofmann DK, Kremer BP (1981) Carbon metabolism and strobilation in Cassiopea andromeda (Cnidaria: Scyphozoa): significance of endosymbiotic dinoflagellates. Mar Biol 65:25–33
Holland BS, Dawson MN, Crow GL, Hofman DK (2004) Global phylogeography of Cassiopea (Schyphozoa: Rhizostomeae): molecular evidence for cryptic species and multiple invasions of the Hawaiian Islands. Mar Biol 145:1119–1128
Kimura M, Barbieri M, Gardner JP, Skurnick J, Cao X, van Riel N, Rizzo MR, Paoliso G, Aviv A (2007) Leukocytes of exceptionally old persons display ultra-short telomeres. Am J Physiol Reg I 293:R2210–R2217
Lian CL, Wadud MA, Geng Q, Shimatani K, Hogetsu T (2006) An improved technique for isolating codominant compound microsatellite markers. J Plant Res 119:415–417
Lindsey J, McGill NI, Lindsey LA, Green DK, Cooke HJ (1991) In vivo loss of telomeric repeats with age in humans. Mutat Res 256:45–48
Luo CX, Yin LF, Koyanagi S, Farman ML, Kusaba M, Yaegashi H (2005) Genetic mapping and chromosomal assignment of Magnaporthe oryzae avirulence genes AvrPik, AvrPiz, and AvrPiz-t controlling cultivar specificity on rice. Phytopathology 95:640–647
Muller WA, Teo R, Frank U (2004) Totipotent migratory stem cells in a hydroid. Dev Biol 275:215–224
Nakagawa S, Gemmell NJ, Burke T (2004) Measuring vertebrate telomeres: applications and limitations. Mol Ecol 13:2523–2533
Nanda I, Schrama D, Feichtinger W, Haaf T, Schartl M, Schmid M (2002) Distribution of telomeric (TTAGGG)n sequences in avian chromosomes. Chromosoma 111:215–227
Nomoto Y, Hirai M, Ueshima R (2001) Cloning of molluscan telomere DNA with (TTAGGG)n repeat and its chromosomal location in the freshwater snail Biwamelania habei. Zool Sci 18:417–422
Ojimi MC, Isomura N, Hidaka M (2009) Telomerase activity is not related to life history stage in the jellyfish Cassiopea sp. Comp Biochem Physiol A 152:240–244
Sfeir AJ, Shay JW, Wright WE (2005) Fine-tuning the chromosome ends—the last base of human telomeres. Cell Cycle 4:1467–1470
Sinclair CS, Richmond RH, Ostrander GK (2007) Characterization of the telomere regions of scleractinian coral, Acropora surculosa. Genetica 129:227–233
Watanabe H, Hoang VT, Mattner R, Holstein TW (2009) Immortality and the base of multicellular life: lessons from cnidarian stem cells. Semin Cell Dev Biol 20:1114–1125
Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW (1996) Telomerase activity in human germline and embryonic tissues and cells. Dev Genet 18:173–179
Yoshida K, Fujisawa T, Hwang JS, Ikeo K, Gojobori T (2006) Degeneration after sexual differentiation in hydra and its relevance to the evolution of aging. Gene 385:64–70
Zielke S, Bodnar A (2010) Telomeres and telomerase activity in scleractinian corals and Symbiodinium spp. Biol Bull 218:113–121
Acknowledgments
We thank Dr. N. Isomura for helpful suggestions at an early phase of this study. This study was supported by Grants-in-Aid for Scientific Research No. 20570093 (MH) from the Ministry of Education, Culture, Sports, Science and Technology, Japan and the twenty-first century Center of Excellence (COE) program of the University of the Ryukyus. We would like to thank anonymous reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. H. Richmond.
Rights and permissions
About this article
Cite this article
Ojimi, M.C., Hidaka, M. Comparison of telomere length among different life cycle stages of the jellyfish Cassiopea andromeda . Mar Biol 157, 2279–2287 (2010). https://doi.org/10.1007/s00227-010-1495-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-010-1495-4