Abstract
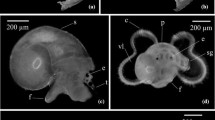
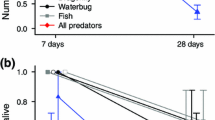
Predator-induced cloning (asexual reproduction), with reduced size as consequence of cloning, suggests a novel adaptation to the threat of predation. Although cloning is a common reproductive strategy of many plants and animals, cloning in response to stimuli from predators has, at present, been documented only in the larvae (plutei) of the sand dollar, Dendraster excentricus. Other studies report larval cloning in echinoderms under optimal conditions of food and temperature. A burst of asexuality should be favored when environmental conditions are conducive to growth, but it is less clear that cloning is advantageous when conditions indicate risk from predators. This study tested the hypothesis that the small size of predator-induced clones reduces vulnerability during encounters with planktivorous fish. Successful cloning was inferred from an increase in larval density, a reduction in larval size and stage, and some direct observations of budding. All clones were smaller than uncloned sibling larvae, suggesting an advantage against visual predators. Pair-wise predation trials demonstrated that planktivorous fish ate more uncloned sibling plutei than small clones. These results offer a new ecological context for asexual reproduction: rapid size reduction as a defense. If the identifiable cues for cloning in echinoderm larvae (food and predators) are linked in nature, then larval cloning may be a response to a single ecological scenario rather than two separate and unrelated conditions.





Similar content being viewed by others
References
Allen JD (2008) Size-selective predation on marine invertebrate larvae. Biological Bulletin 214
Balser EJ (1998) Cloning by ophiuroid echinoderm larvae. Biol Bull 194:187–193
Benard MF (2004) Predator-induced phenotypic plasticity in organisms with complex life histories. Ann Rev Ecol Evol Syst 35:651–673
Bergkvist J, Selander E, Pavia H (2008) Induction of toxin production in dinoflagellates: the grazer makes a difference. Oecologia 156:147–154
Bollens SM, Frost BW, Thoreson DS, Watts SJ (1992) Diel vertical migration in zooplankton—field evidence in support of the predator avoidance hypothesis. Hydrobiologia 234:33–39
Bosch I, Rivkin RB, Alexander SP (1989) Asexual reproduction by oceanic planktotrophic echinoderm larvae. Nature 337:169–170
Bronmark C, Miner JG (1992) Predator-induced phenotypical change in body morphology in crucian carp. Science 258:1348–1350
Brooks JL, Dodson SI (1965) Predation, body size, and composition of plankton. Science 150:28–35
Chen MS (2008) Inducible direct plant defense against insect herbivores: a review. Insect Sci 15:101–114
Craig SF, Slobodkin LB, Wray GA, Biermann CH (1997) The ‘paradox’ of polyembryony: a review of the cases and a hypothesis for its evolution. Evol Ecol 11:127–143
Cronin G, Hay ME (1996) Induction of seaweed chemical defense by amphipod grazing. Ecology 77:2287–2301
De Robertis A, Jaffe JS, Ohman MD (2000) Size-dependent visual predation risk and the timing of vertical migration in zooplankton. Limnol Oceanogr 45
DeMeester L, Dawidowicz P, van Gool E, Loose CJ (1999) Predator-induced behavior of zooplankton: Depth selection behavior and diel vertical migration. In: Tollrian R, Harvell CD (eds) The ecology and evolution of inducible defenses. Princeton University Press, Princeton, pp 160–176
Eaves AA, Palmer AR (2003) Widespread cloning in echinoderm larvae. Nature 425:146
Folt CL, Burns CW (1999) Biological drivers of zooplankton patchiness. Trends Ecol Evol 14:300–305
Forward RB, Rittschof D (1999) Brine shrimp larval photoresponses involved in diel vertical migration: activation by fish mucus and modified amino sugars. Limnol Oceanogr 44:1904–1916
Forward RB, Rittschof D (2000) Alteration of photoresponses involved in diel vertical migration of a crab larva by fish mucus and degradation products of mucopolysaccharides. J Exp Mar Biol Ecol 245:277–292
Genin A, Jaffe JS, Reef R, Franks PJS (2005) Swimming against the flow: a mechanism of zooplankton aggregation. Science 308:860–862
Gill AB, Hart PJB (1998) Stomach capacity as a directing factor in prey size selection of three-spined stickleback. J Fish Biol 53:897–900
Grünbaum D (2001) Predicting availability to consumers of spatially and temporally variable resources. Hydrobiologia 480:175–191
Harvell CD (1986) The ecology and evolution of inducible defenses in a marine bryozoan. Am Nat 128:810–823
Highsmith RC (1982) Induced settlement and metamorphosis of sand dollar (Dendraster excentricus) larvae in predator-free sites: adult sand dollar beds. Ecology 63:329–337
Iglesias C, Mazzeo N, Goyenola G, Fosalba C, De Mello FT, Garcia S, Jeppesen E (2008) Field and experimental evidence of the effect of Jenynsia multidentata, a small omnivorous-planktivorous fish, on the size distribution of zooplankton in subtropical lakes. Freshw Biol 53:1797–1807
Jaeckle WB (1994) Multiple-modes of asexual reproduction by tropical and subtropical sea star larvae—an unusual adaptation for genet dispersal and survival. Biol Bull 186:62–71
Jang MH, Ha K, Joo G, Takamura N (2003) Toxic production of cyanobacteria is increased by exposure to zooplankton. Freshw Biol 48:1540–1550
Knott KE, Balser EJ, Jaeckle WB, Wray GA (2003) Identification of asteroid genera with species capable of larval cloning. Biol Bull 204:246–255
Levins R (1968) Evolution in changing environments. Princeton University Press, Princeton
Lima SL, Dill LM (1990) Behavioural decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640
Marshall DJ, Keough MJ (2008) The evolutionary ecology of offspring size in marine invertebrates. Adv Mar Biol 53:1–60
McCollum SA, Leimberger JD (1997) Predator-induced morphological changes in an amphibian: predation by dragonflies affects tadpole shape and color. Oecologia 109:615–621
McDonald K, Vaughn D (2010) An abrupt change in food environment induces cloning in Dendraster excentricus plutei. Biol Bull (in press)
Menden-Deuer S (2006) Individual foraging behaviors and population distributions of planktonic predators to phytoplankton thin layers. Limnol Oceanogr 45:569–579
Menden-Deuer S (2008) Spatial and temporal characteristics of plankton-rich layers in a shallow, temperate fjord. Mar Ecol Prog Ser 355:21–30
Metaxas A (2001) Behaviour in flow: perspectives on the distribution and dispersion of meroplanktonic larvae in the water column. Can J Fish Aquat Sci 58:86–98
Metaxas A, Young CM (1998) Response of echinoid larvae to food patches of different algal densities. Mar Biol 130:433–445
Miner BG (2004) Evolution of feeding structure plasticity in marine invertebrate larvae: a possible trade-off between arm length and stomach size. J Exp Mar Biol Ecol 315:117–125
O’Brien WJ (1979) The predator-prey interaction of planktivorous fish and zooplankton. Am Sci 67:572–581
Padilla DK, Adolph SC (1996) Plastic inducible morphologies are not always adaptive: the importance of time delays in a stochastic environment. Evol Ecol 10:456
Palmer AR (1990) Effect of crab effluent and scent of damaged conspecifics on feeding, growth, and shell morphology of the Atlantic dogwhelk Nucella lapillus. Hydrobiologia 193:155–182
Pennington JT, Rumrill SS, Chia FS (1986) Stage specific predation upon embryos and larvae of the Pacific sand dollar, Dendraster excentricus, by 11 species of common zooplankton predators. Bull Mar Sci 39:234–240
Ruehl CB, DeWitt TJ (2005) Trophic plasticity and fine-grained resource variation in populations of western mosquitofish, Gambusia affinis. Evol Ecol Res 7:801–819
Schabetsberger R, Luger MS, Drozdowski G, Jagsch A (2009) Only the small survive: monitoring long-term changes in the zooplankton community of an Alpine lake after fish introduction. Biol Invasions 11:1335–1345
Selander E, Thor P, Toth GB, Pavia H (2006) Copepods induce paralytic shellfish toxin production in marine dinoflagellates. Proc R Soc Lond 273:1673–1680
Seuront L, Schmitt F, Lagadeuc Y (2001) Turbulence intermittency, small-scale phytoplankton patchiness and encounter rates in the plankton: where do we go from here? Deep Sea Res 48:1199–1215
Sewell MA, Cameron MJ, McArdle B (2004) Developmental plasticity in larval development in the echinometrid sea urchin Evechinus chloroticus with varying food ration. J Exp Mar Biol Ecol 309:219–237
Sih A (2004) A behavioral ecological view of phenotypic plasticity. In: Dewitt TJ, Scheiner SM (eds) Phenotypic plasticity: functional and conceptual approaches. Oxford University Press, New York, pp 112–125
Strathmann RR (1971) The behavior of planktotrophic echinoderm larvae: mechanisms, regulation, and rates of suspension feeding. J Exp Mar Biol Ecol 6:109–160
Strathmann MF (1987) Reproduction and development of marine invertebrates of the Northern Pacific Coast. University of Washington Press, Seattle and London
Strathmann RR (1996) Are planktonic larvae of marine benthic invertebrates too scarce to compete within species? Oceanol Acta 19:399–407
Strathmann RR, Fenaux L, Strathmann MF (1992) Heterochronic developmental plasticity in larval sea urchins and its implications for evolution of nonfeeding larvae. Evolution 46:972–986
Suchman CL, Sullivan BK (2000) Effect of prey size on vulnerability of copepods to predation by scyphomedusae Aurelia aurita and Cyanea sp. J Plankton Res 22:2289–2306
Tollrian R, Harvell CD (1999) The ecology and evolution of inducible defenses. Princeton University Press, Princeton
Trussell GC (1996) Phenotypic plasticity in an intertidal snail: the role of a common crab predator. Evolution 50:448–454
Van Der Stap I, Vos M, Verschoor AM, Helmsing NR, Mooij WM (2007) Induced defenses in herbivores and plants differentially modulate a trophic cascade. Ecology 88:2474–2481
Van Donk E (2007) Chemical information transfer in freshwater plankton. Ecol Inf 2:112–120
Vaughn D (2007) Predator-induced morphological defenses in marine zooplankton: a larval case study. Ecology 88:1030–1039
Vaughn D (2009) Predator-induced larval cloning in the sand dollar Dendraster excentricus: might mothers matter? Biol Bull 217:103–114
Vaughn D, Strathmann RR (2008) Predators induce cloning in echinoderm larvae. Science 319:1503
Vickery MCL, McClintock JB (1998) Regeneration in metazoan larvae. Nature 394:140
Vickery MS, McClintock JB (2000) Effects of food concentration and availability on the incidence of cloning in planktotrophic larvae of the sea star Pisaster ochraceus. Biol Bull 199:298–304
Vos M, Verschoor AM, Kooi BW, Wackers FL, DeAngelis DL, Mooij WM (2004) Inducible defenses and trophic structure. Ecology 85:2783–2794
Vos M, Vet LEM, Wackers FL, Middleburg JJ, van der Putten WH, Mooji WM, Heip CHR, Van Donk E (2006) Infochemicals structure marine, terrestrial and freshwater food webs: implications for ecological informatics. Ecol Inf 1:23–32
Warkentin KM (1995) Adaptive plasticity in hatching age: a response to predation risk trade-offs. Proc Nat Acad Sci USA 92:3507–3510
Werner EE, Hall DJ (1974) Optimal foraging and the size selection of prey by bluegill sunfish (Lepomis macrochirus). Ecology 55:1042–1052
Woodson CB, McManus MA (2007) Foraging behavior can influence dispersal of marine organisms. Limnol Oceanogr 52:2701–2709
Woodson CB, Webster DR, Weissburg MJ, Yen J (2005) Response of copepods to physical gradients associated with structure in the ocean. Limnol Oceanogr 50:1552–1564
Acknowledgments
NSF grant OCE0623102 to R. R. Strathmann, the Friday Harbor Laboratories, and the ARCS Foundation supported this study. I thank R. R. Strathmann, M. F. Strathmann, E. Dusek, R. Kodner, and K. Vaughn for advice and assistance and two anonymous reviewers for comments that improved the manuscript. I also thank P. Kitaeff and the 2008 class in Functional Morphology and Ecology of Marine Fishes at the FHL for collection of the predatory fish used in this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Rights and permissions
About this article
Cite this article
Vaughn, D. Why run and hide when you can divide? Evidence for larval cloning and reduced larval size as an adaptive inducible defense. Mar Biol 157, 1301–1312 (2010). https://doi.org/10.1007/s00227-010-1410-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-010-1410-z