Abstract
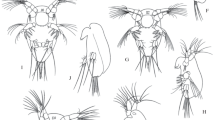
Close to 50 species of marine Calanoid copepods have been reported to produce diapause eggs (Engel and Hirche in J Plankton Res 26:1083–1093, 2004); eggs that are viable but require a refractory phase before they hatch, sometimes after months. Diapause eggs are often described as morphologically different with respect to egg membrane ultrastructure and having a thicker egg shell with surface ornamentation as opposed to the smooth shell found in subitaneous eggs that hatch within days (Belmonte in J Mar Syst 15:35–39, 1998; Chen and Marcus in Mar Biol 127:587–597, 1997; Castro-Longoria in Crustaceana 74:225–236, 2001). Egg production rates, egg surface ornamentation, and hatching success were monitored in large aquaculture fish enclosures during winter with close to zero water temperatures (N57°). Surprisingly, all female copepods (Acartia spp.—presumably A. tonsa, and Centropages hamatus) produced eggs all through the winter with no obvious pattern with respect to light, temperature and food availability, and no diapause eggs were observed. However, individual females produced several categories of eggs with or without surface spines even within the same egg batch as evidenced by scanning electron microscopy (SEM). Four egg categories were distinguishable: ‘no spines’, smooth eggs; ‘short spines’, 5–15 μm long; ‘truncated spines’, with the spine tips cut-off <10 μm long; and ‘long spines’, up to 30 μm long. All egg categories remained unchanged with respect to surface structures from when we took them out of the incubation bottles until they hatched. In general, the frequency of ‘no spines’ was 10–40%, and most eggs were ornamented with ‘short-’ or ‘long spines’. Further, a given egg can be ornamented with all types of surface spines simultaneously, which might even be a fifth egg category. The different egg categories were all able to hatch within days when exposed to normoxic conditions suggesting that they were subitaneous.










Similar content being viewed by others
References
Alekseev VR, De Stasio B, Gilbert JJ (2007) Diapause in aquatic invertebrates; Theory and human use. Springer, Netherlands, p 257 ISBN 978-1-4020-5679-6
Belmonte G (1992) Diapause egg-production in Acartia (Paracartia) latisetosa (Crustacea, Copepoda, Calanoida). Bollettino di Zoologia 59:363–366
Belmonte G (1998) The egg morphology of 7 Acartiidae species: a preliminary survey of the ootaxonomy of calanoids. J Mar Syst 15:35–39
Belmonte G, Puce M (1994) Morphological aspects of subitaneous and resting eggs from Acartia josephinae (Calanoida). Hydrobiologia 292–293:131–135
Belmonte G, Miglietta A, Rubino F, Boero F (1997) Morphological convergence of resting stages of planktonic organisms: a review. Hydrobiologia 355:159–165
Blades-Eckelbarger PI, Marcus NH (1992) The origin of cortical vesicles and their role in egg envelope formation in the “spiny” eggs of a calanoid copepod, Centropages velificatus. Biol Bull 182:41–53
Camus T, Zeng C (2009) The effects of stocking density on egg production and hatching success, cannibalism rate, sex ratio and population growth of the tropical calanoid copepod Acartia sinjiensis. Aquaculture 287:145–151
Castellani C, Lucas IAN (2003) Seasonal variation in egg morphology and hatching success in the calanoid copepods Temora longicornis, Acartia clausi and Centropages hamatus. J Plankton Res 25:527–537
Castro-Longoria E (2001) Comparative observations on the external morphology of subitaneous and diapause eggs of Acartia species from Southampton water. Crustaceana 74:225–236
Chen F, Marcus NH (1997) Subitaneous, diapause, and delayed-hatching eggs of planktonic copepods from the northern Gulf of Mexico: morphology and hatching success. Mar Biol 127:587–597
Couch KM, Downes M, Burns CW (2001) Morphological differences between subitaneous and diapause eggs of Boeckella triarticulata (Copepoda: Calanoida). Freshw Biol 46:925–933
Dahms HU (1995) Dormancy in the copepoda–an overview. Hydrobiologia 306:199–211
Dharani G, Altaff K (2004) Ultra structure of subitaneous and diapausing eggs of planktonic copepod Sinodiaptomus (Rhinediaptomus) indicus. Curr Sci 87:109–112
Drillet G, Goetze E, Jepsen PM, Højgaard JK, Jørgensen NOG, Hansen BW (2008a) Strain-specific vital rates in four Acartia tonsa cultures I: strain origin, genetic differentiation and egg survivorship. Aquaculture 280:109–116
Drillet G, Jepsen PM, Højgaard JK, Jørgensen NOG, Hansen BW (2008b) Strain-specific vital rates in four Acartia tonsa cultures II: life history traits and biochemical contents of eggs and adults. Aquaculture 279:47–54
Dumont HJ, Nandini S, Sarma SSS (2002) Cyst ornamentation in aquatic invertebrates: a defence against egg-predation. Hydrobiologia 486:161–167
Engel M, Hirche H-J (2004) Seasonal variability and inter-specific differences in hatching of calanoid copepod resting eggs from sediments of the German Bight (North Sea). J Plankton Res 26:1083–1093
Engell-Sørensen K, Støttrup JG, Holmstrup M (2004) Rearing of flounder (Platichtys flesus) juveniles in semi-extensive systems. Aquaculture 230:475–491
Flinkman J, Vuorinen I, Christiansen M (1994) Calanoid copepod eggs survive passage through fish digestive tracts. ICES J Mar Sci 51:127–129
Grice GD, Marcus NH (1981) Dormant eggs of marine copepods. Oceanogr Mar Biol Ann Rev 19:125–140
Hairston NG Jr, Olds EJ (1987) Population differences in the turning of diapauses: a test of hypothesis. Oecologia 71:339–344
Hansen PJ, Bjørnsen PK, Hansen BW (1997) Zooplankton grazing and growth: Scaling within the 2–2,000 μm body size range. Limnol Oceanogr 42:687–704
Hansen BW, Drillet G, Kozmér A, Madsen KV, Pedersen MF, Sørensen TF (2009) Temperature effect on hatching of copepod eggs: Does acclimation matters? J Plankton Res (In revision)
Hirose E, Toda H, Saito Y, Watanabe H (1992) Formation of the multiple-layered fertilization envelope in the embryo of Calanus sinicus Brodsky (Copepoda: Calanoida). J Crustac Biol 12:186–192
Holmstrup M, Overgaard J, Sørensen TF, Drillet G, Hansen BW, Ramløv H, Engell-Sørensen K (2006) Influence of storage conditions on viability of quiescent copepod eggs (Acartia tonsa Dana): effects of temperature, salinity and anoxia. Aquac Res 37:625–631
Hubble SK, Kirby RR (2007) Transmission electron microscopy of marine crustacean eggs. Crustaceana 80:739–745
Ianora A, Santella L (1991) Diapause embryos in the neustonic copepod Anomalocera patersoni. Mar Biol 108:387–394
Katajisto T (2003) Development of Acartia bifilosa (Copepoda: Calanoida) eggs in the northern Baltic Sea with special reference to dormancy. J Plankton Res 25:357–364
Katajisto T (2006) Benthic resting eggs in the life cycles of calanoid copepods in the northern Baltic Sea. Walther and Andrée de Nottbeck Foundation Scientific Reports No. 29. PhD-thesis, Faculty of Biosciences of the University of Helsinki
Kiørboe T, Nielsen TG (1994) Regulation of zooplankton biomass and production in a temperate, coastal ecosystem. 1. Copepods. Limnol Oceanogr 39:493–507
Koga F (1968) On the pelagic eggs of copepoda. J Oceanogr Soc Japan 24:16–20
Li SJ, Chen F, Wang GZ (1989) Studies on the feature of eggs and their hatching rates of some planktonic copepods in Xiamen waters. J Xiamen Univ (Nat Sci) 28:538–543 (in Chinese with English abstract)
Marcus NH (1996) Ecological and evolutionary significance of resting eggs in marine copepods: past, present, and future studies. Hydrobiologia 320:141–152
Nielsen P, Mortensen J, Vismann B, Hansen BW (2006) Physiological tolerance of marine calanoid copepod eggs to sulphide. Mar Ecol Prog Ser 328:171–182
Santella L, Ianora A (1990) Subitaneous and diapause eggs in Mediterranean populations of Pontella mediterranea (Copepoda: Calanoida): a morphological study. Mar Biol 105:83–90
Sichlau MH, Hansen JLS, Andersen TJ, Hansen BW (2009) Temporal and spatial distribution and mortality of diapause eggs from calanoid copepods in sediment profiles at 55°N. Mar Ecol Prog Ser (In revision)
Sørensen TF, Drillet G, Engell-Sørensen K, Hansen BW, Ramløv H (2007) Production and biochemical composition of eggs from neritic calanoid copepods reared in large outdoor tanks (Limfjord, Denmark). Aquaculture 263:84–96
Toda H, Hirose E (1991) SEM and TEM observations on egg membranes of two types of Calanus sinicus eggs. Bull Plankton Soc Japan, Special volume, 4th International conference on Copepoda, pp 613–617
Uye S (1985) Resting egg production as a life history strategy of marine planktonic copepods. Bull Mar Sci 37:440–449
Acknowledgments
We are greatly indebted to the aquaculture industry Maximus A/S by Jane Sørensen and Anders Thinggaard Pedersen for access to their fish enclosures. We also want to thank Bioconsult A/S by Kirsten Engell-Sørensen and Poul Sebach for zooplankton analysis and Danish Meteorological Institute for access to light data. We want to thank Dr. Hans Ramløv (HR) and the anonymous reviewers for improving earlier drafts of the manuscript. This study was supported by EU-CRAFT project POCEFF no. Q5CR-2002-72468 to HR and BWH.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Rights and permissions
About this article
Cite this article
Hansen, B.W., Drillet, G., Kristensen, R.M. et al. Production, hatching success and surface ornamentation of eggs of calanoid copepods during a winter at 57°N. Mar Biol 157, 59–68 (2010). https://doi.org/10.1007/s00227-009-1295-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-009-1295-x