Abstract
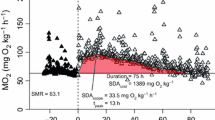
Predictions of short and long term changes in Sepia officinalis metabolism are useful, since this species is both economically important for aquaculture and also is an ideal experimental laboratory organism. In this study standard and routine oxygen consumption rates of newly hatched and juvenile laboratory raised cuttlefish S. officinalis ranging between 0.04 and 18.48 g dry body mass (Dm), were measured over a range of temperatures (10, 15, 20 and 25°C). The mass exponent (b) ranged between 0.706 and 0.992 for standard oxygen consumption and between 0.694 and 0.990 for routine oxygen consumption. Oxygen consumption scaled allometrically (b = 0.7) with body mass for cuttlefish <2 g Dm and isometrically (b = 1) thereafter. No significant differences were apparent amongst the slopes of oxygen consumption and body mass at different temperatures for standard and routine oxygen consumption. However, the intercepts differed significantly amongst the regression lines, indicating a significant effect of temperature on the magnitude of oxygen consumption. The combined effect of temperature (T) and dry body mass (Dm) are best described by the following equations: cuttlefish <2 g, MO2 = 0.116Dm0.7111.086T and >2 g, MO2 = 0.076Dm0.9831.091T for standard oxygen consumption; cuttlefish <2 g, MO2 = 0.538Dm0.7291.057T and >2 g, MO2 = 0.225Dm0.9621.081T for routine oxygen consumption. Using these equations it was estimated that a cuttlefish of 1 g Dm held at 20°C, eating 5% Dm day−1 and undergoing standard and routine metabolism consumes 21.3 and 35.4%, respectively of its total daily energy intake. Juvenile cuttlefish (3.32–5.08 g Dm) held at 15°C and deprived of food for 27 days maintained a stable standard oxygen consumption rate for the first 6 days following starvation. By the 18th day without food, oxygen consumption rate had declined by 53% and further declined to 65% below the standard oxygen consumption rate on the 27th day. Upon resumption of feeding, the respiration rate returned immediately to the initial level prior to food deprivation. The present study defines the basic energy requirements and general physiological state of young cuttlefish at temperatures of 10–25°C with and without food.




Similar content being viewed by others
References
Belman BW (1978) Respiration and effects of pressure on the mesopelagic vertically migrating squid Histioteuthis heteropsis. Limnol Oceanogr 23:735–739
Bokma F (2004) Evidence against universal metabolic allometry. Funct Ecol 18:184–187
Boletzky SV (1983) Sepia officinalis. In: Boyle PR (ed) Cephalopod life cycles, vol I. Academic Press, London, pp 31–52
Boletzky Sv, Hanlon RT (1983) A review of the laboratory maintenance, rearing and culture of cephalopod molluscs. Mem Nat Mus Vict 44:147–187
Brett JR, Groves TDD (1979) Physiological energetics. In: Hoar WS, Randall DJ, Brett JR (eds) Fish physiology vol VIII. Academic Press, New York, pp 279–352
Castro BG, Garrido JL, Sotelo CG (1992) Changes in composition of digestive gland and mantle muscle of the cuttlefish Sepia officinalis during starvation. Mar Biol 114:11–20
Cerezo Valverde J, García García B (2004) Influence of body mass and temperature on post-prandial oxygen consumption of common octopus (Octopus vulgaris). Aquaculture 233:599–613
Cook JT, Sutterlin AM, McNiven MA (2000) Effect of food deprivation on oxygen consumption and body composition of growth-enhanced transgenic Atlantic salmon (Salmosalar). Aquaculture 188:47–63
Daly HI, Peck LS (2000) Energy balance and cold adaptation in the octopus Pareledone charcoti. J Exp Mar Biol Ecol 245:197–214
Darveau CA, Suarez RK, Andrews RD, Hochachka PW (2002) Allometric cascade as a unifying principle of body mass effects on metabolism. Nature 417:166–170
DeMont ME, O’Dor RK (1984) The effects of activity, temperature and mass on the respiratory metabolism of the squid, Illex illecebrosus. J Mar Biol Ass UK 64:535–543
Dodds PS, Rothman DH, Weitz JS (2001) Re-examination of the 3/4 law of metabolism. J Theor Biol 209:9–27
Domingues PM, Kingston T, Sykes A, Andrade JP (2001) Growth of young cuttlefish, Sepia officinalis (Linnaeus 1758) at the upper end of the biological distribution temperature range. Aquac Res 32:923–930
Elliot JM, Davison W (1975) Energy equivalents of oxygen consumption in animal energetics. Oecologia 19:195–201
FAO (Food and Agriculture Organisation) (2005) https://doi.org/www.fao.org
Fidhiany L, Winckler K (1998) Influence of body mass, age and maturation on specific oxygen consumption in a freshwater cichlid fish, Cichlasoma nigrofasciatum (Günther, 1869). Comp Biochem Physiol A 119:613–619
Forsythe JW, De Rusha RH, Hanlon RT (1994) Growth, reproduction and life span of Sepia officinalis (Cephalopoda: Mollusca) cultured through seven consecutive generations. J Zool 233:175–192
Fry FEJ (1971) The effect of environmental factors on the physiology of fish. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 1. Academic Press, New York, pp 1–98
Green EJ, Carritt DE (1967) New tables for oxygen saturation of seawater. J Mar Res 25:140–147
Grigoriou P (2005) The growth and physiology of the common cuttlefish Sepia officinalis (L.) (Mollusca: Cephalopoda). PhD Thesis. University of Wales, Bangor
Grigoriou P, Richardson CA (2004) Aspects of the growth of cultured cuttlefish Sepia officinalis (Linnaeus 1758). Aquac Res 35:1141–1148
Grigoriou P, Richardson CA (2008) The effect of ration size, temperature and body mass on specific dynamic action of the common cuttlefish Sepia officinalis. Mar Biol 154:1085–1095
Hogendoorn H (1983) Growth and production of the African catfish, Clarias lazera (C. and V.): III. Bioenergetic relations of body mass and feeding level. Aquaculture 35:1–17
Jobling M (1983) Towards an explanation of specific dynamic action (SDA). J Fish Biol 23:549–555
Johansen K, Brix O, Kornerup S, Lykkeboe G (1982) Factors affecting O2-uptake in the cuttlefish, Sepia officinalis. J Mar Biol Ass UK 62:187–191
Kanwisher J (1959) Polarographic oxygen electrode. Limnol Oceanogr 4:210–217
Katsanevakis S, Stephanopoulou S, Miliou H, Moraitou-Apostolopoulou M, Verriopoulos G (2005) Oxygen consumption and ammonia excretion of Octopus vulgaris (Cephalopoda) in relation to body mass and temperature. Mar Biol 146:725–732
Kinoshita J, Hiromi J, Kadota S (1997) Do respiratory metabolic rates of the scyphomedusa Aurelia aurita scale isometrically throughout ontogeny in a sexual generation? Hydrobiologia 347:51–55
Louw GN (ed) (1993) Physiological animal ecology. Longman Group UK Limited Harlow, London
Lu YT, Blake NJ, Torres JJ (1999) Oxygen consumption and ammonia excretion of larvae and juveniles of the bay scallop, Argopectenirradiansconcentricus (Say). J Shellfish Res 18:419–423
Lucas A (ed) (1996) Bioenergetics of aquatic animals, English edition. Taylor and Francis Ltd, London
Mangold-Wirz K (1963) Biologie des céphalopodes benthiques et nectoniques de la Mer Catalane. Vie et Milieu 13:1–285
Mehner T, Wieser W (1994) Energetics and metabolic correlates of starvation in juvenile perch (Perca fluviatilis). J Fish Biol 45:325–333
Melzner F, Bock C, Pörtner HO (2007) Allometry of thermal limitation in the cephalopod Sepia officinalis. Comp Biochem Physiol A 146:149–154
Mill PJ (ed) (1972) Respiration in the invertebrates. St. Martin’s Press, London
Montuori A (1913) Les processus oxydatifs chez les animaux marins en rapport avec la loi de superficie. Arch Ital Biol 59:213–234
Nixon M (1987) Cephalopod diets. In: Boyle PR (ed) Cephalopod life cycles, vol II: comparative reviews. Harcourt Brace Jovanovich, London, pp 201–220
O’Dor RK, Wells MJ (1987) Energy and nutrient flow. In: Boyle PR (ed) Cephalopod life cycles, vol II: comparative reviews. Harcourt Brace Jovanovich, London, pp 109–133
Post JR, Lee JA (1996) Metabolic ontogeny of teleost fishes. Can J Fish Aquat Sci 53:910–923
Raffy A, Ricart R (1939) Influence des variations de salinité sur la consommation d’oxygène par les Céphalopodes. CR Acad Sci Paris 208:671–673
Segawa S (1991) Body size and oxygen consumption rate of the oval squid Sepioteuthis lessoniana. Nippon Suisan Gakk 57:1651–1656
Segawa S (1995) Effect of temperature on oxygen consumption of juvenile oval squid Sepioteuthis lessoniana. Fisheries Sci 61:743–746
Segawa S, Hanlon RT (1988) Oxygen consumption and ammonia excretion rates in Octopus maya, Loligo forbesi and Lolliguncula brevis (Mollusca: Cephalopoda). Mar Behav Physiol 13:389–400
Szaniawska A (1983) Seasonal changes in energy content of Crangon crangon L. (Crustacea, Decapoda). Pol Arch Hydrobiol 30:45–56
von Bertalanffy L (1957) Quantitative laws in metabolism and growth. Q Rev Biol 32:217–231
Wachter BD, Wolf G, Richard A, Decleir W (1988) Regulation of respiration during juvenile development of Sepia officinalis (Mollusca: Cephalopoda). Mar Biol 97:365–371
Wells RMG (ed) (1980) Invertebrate respiration. Arnold E, London
Wells MJ, O’Dor RK, Mangold K, Wells J (1983a) Diurnal changes in activity and metabolic rate in Octopus vulgaris. Mar Behav Physiol 9:275–287
Wells MJ, O’Dor RK, Mangold K, Wells J (1983b) Feeding and metabolic rate in octopus. Mar Behav Physiol 9:305–317
Wells MJ, O’Dor RK, Mangold K, Wells J (1983c) Oxygen consumption in movement by Octopus. Mar Behav Physiol 9:289–303
West GB, Brown JH, Enquist BJ (1997) A general model for the origin of allometric scaling laws in biology. Science 276:122–126
Wolf G, Verheyen E, Vlaeminck A, Lemaire J, Decleir W (1985) Respiration of Sepia officinalis during embryonic and early juvenile life. Mar Biol 90:35–39
Acknowledgments
The authors express their gratitude to the Alexander S. Onassis Public Benefit Foundation and to the School of Ocean Sciences, University of Wales for funding this project. Special thanks to Dr. A. B. Yule for his assistance with the statistical analysis, his criticism and advice in laboratory work and for loan of his equipment. Many thanks to Berwyn Roberts and Gwyn Hughes for help in obtaining the live food. We would also like to thank Dr. Eva Chatzinikolaou for her helpful and valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. P. Grassle.
Rights and permissions
About this article
Cite this article
Grigoriou, P., Richardson, C.A. Effect of body mass, temperature and food deprivation on oxygen consumption rate of common cuttlefish Sepia officinalis . Mar Biol 156, 2473–2481 (2009). https://doi.org/10.1007/s00227-009-1272-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-009-1272-4