Abstract
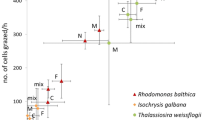
Copepods play a central role in marine food webs as grazers of plankton and as key prey for many predators. Therefore, quantifying their specific trophic interactions is critical for understanding the role of copepods in ocean processes. However, because of methodological constraints, it remains difficult to investigate in situ copepod feeding without reliance on laborious intrusive and potentially biased incubation approaches. Recent advances in PCR-based methodologies have demonstrated the feasibility of directly identifying copepod diets based on prey DNA sequences. Yet, obtaining quantitative information from these approaches remains challenging. This study presents results of systematic efforts to develop a quantitative PCR (qPCR) assay targeted to 18S rRNA gene fragments to estimate copepod gut content of specific species of prey algae. These results were first compared to gut content estimates based on fluorescence in the copepod Calanus finmarchicus fed monocultures of two different microalgae species in controlled laboratory studies. In subsequent field studies, we compared feeding rates obtained by microscopy and qPCR for Temora longicornis and Acartia clausi feeding on the haptophyte Phaeocystis globosa in natural blooms. These investigations demonstrate a semi-quantitative relationship between gut content estimates derived from qPCR, gut pigment, and direct microscopy. However, absolute estimates of gut content based on qPCR methodology were consistently lower than expected. This did not appear to be explained by the extraction methods used, or interference by non-target (predator) DNA in the PCR reactions, instead suggesting digestion of prey-specific nucleic acids. Furthermore, the 18S rDNA target gene copy number of the phytoplankton varied with growth phase. Nonetheless, when prey target gene copy number in the ambient water is quantified, the qPCR-approach can be compared to other methods, and then used to semi-quantitatively estimate relative copepod grazing on specific prey in situ without involving further incubations. A distinct advantage of a DNA-based molecular approach compared to gut fluorescence and direct microscopic observation, is the ability to detect non-pigmented and macerated prey. Future studies should aim to correct for breakdown in prey DNA and perform extensive calibrations to other methods in order to achieve a quantitative measure of feeding rates in situ.






Similar content being viewed by others
References
Antajan E, Chretiennot-Dinet M-J, Leblanc C, Daro M-H, Lancelot C (2004) 19′-Hexanoyloxyfucoxanthin may not be the appropriate pigment to trace occurrence and fate of Phaeocystis: the case of P. globosa in Belgian coastal waters. J Sea Res 52:165–177
Atkinson A (1996) Subantarctic copepods in an oceanic, low chlorophyll environment: ciliate predation, food selectivity and impact on prey populations. Mar Ecol Prog Ser 130:85–96
Båmstedt U, Gifford DJ, Irigoien X, Atkinson A, Roman M (2000) Feeding. In: Harris R, Wiebe P, Lenz J, Skjoldal HR, Huntley M (eds) ICES zooplankton methodology manual. Academic, London, pp 297–399
Båmstedt U, Nejstgaard JC, Solberg PT (1999) Utilisation of small-sized food algae by Calanus finmarchicus (Copepoda, Calanoida) and the significance of feeding history. Sarsia 84:19–38
Berggreen U, Hansen B, Kiørboe T (1988) Food size spectra, ingestion and growth of the copepod Acartia tonsa during development: implications for determination of copepod production. Mar Biol 99:341–352
Billones RG, Tackx MLM, Flachier AT, Zhu L, Daro MH (1999) Image analysis as a tool for measuring particulate matter concentrations and gut content, body size, and clearance rates of estuarine copepods: validation and application. J Mar Syst 22:179–194
Blankenship LE, Yayanos AA (2005) Universal primers and PCR of gut contents to study marine invertebrate diets. Mol Ecol 14:891–899
Brunet M, Arnaud J, Mazza J (1994) Gut structure and digestive cellular processes in marine crustacea. Oceanography and marine biology. Annu Rev 32:335–367
Buffan-Dubau E, de Wit R, Castel J (1996) Feeding selectivity of the harpacticoid copepod Canuella perplexa in benthic muddy environments demonstrated by HPLC analyses of chlorin and carotenoid pigments. Mar Ecol Prog Ser 137:71–82
Bustillos-Guzman J, Lopez-Cortes D, Mathus ME, Hernandez F (2002) Dynamics of pigment degradation by the copepodite stage of Pseudodiaptomus euryhalinus feeding on Tetraselmis suecica. Mar Biol 140:143–149
Calbet A, Saiz E (2005) The ciliate–copepod link in marine ecosystems. Aquat Microb Ecol 38:157–167
Christoffersen K, Nybroe O, Jürgens K, Hansen M (1997) Measurement of bacterivory by heterotrophic nanoflagellates using immunofluorescence labelling of ingested cells. Aquat Microb Ecol 13:127–134
Cushing DH (1990) Plankton production and year-class strength in fish populations: an update of the match/mismatch hypothesis. Adv Mar Biol 26:249–293
Deagle BE, Eveson JP, Jarman SN (2006) Quantification of damage in DNA recovered from highly degraded samples—a case study on DNA in faeces. Front Zool 3:11
Dutz J, Koski M (2006) Trophic significance of solitary cells of the prymnesiophyte Phaeocystis globosa depends on cell type. Limnol Oceanogr 51:1230–1238
Dyhrman ST, Erdner D, Du JL, Galac M, Anderson DM (2006) Molecular quantification of toxic Alexandrium fundyense in the Gulf of Maine using real-time PCR. Harmful Algae 5:242–250
Fessenden L, Cowles TJ (1994) Copepod predation on phagotrophic ciliates in Oregon coastal waters. Mar Ecol Prog Ser 107:103–111
Fleddum A, Kaartvedt S, Ellertsen B (2001) Distribution and feeding of the carnivorous copepod Paraeuchaeta norvegica in habitats of shallow prey assemblages and midnight sun. Mar Biol 139:719–726
Frischer ME, Lee RF, Sheppard MA, Mauer A, Rambow F, Neumann M, Brofft JE, Wizenmann T, Danforth JM (2006) Evidence for a free-living life stage of the blue crab parasitic dinoflagelate, Hematodinium sp. Harmful Algae 5:548–557
Froneman PW (2004) In situ feeding rates of the copepods, Pseudodiaptomus hessei and Acartia longipatella, in a temperate, temporarily open/closed Eastern Cape estuary. South Afr J Sci 100:577–583
Frost BW (1972) Effect of size and concentration of food particles on the feeding behaviour of the marine planktonic copepod Calanus pacificus. Limnol Oceanogr 17:805–815
Galluzzi L, Penna A, Bertozzini E, Giacobbe MG, Vila M, Garces E, Prioli S, Magnani M (2005) Development of a qualitative PCR method for the Alexandrium spp. (Dinophyceae) detection in contaminated mussels (Mytilus galloprovincialis). Harmful Algae 4:965–1130
Gowing MM, Wishner KF (1992) Feeding ecology of benthopelagic zooplankton on an eastern tropical Pacific seamount. Mar Biol 112:451–467
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum Press, New York, pp 29–60
Guisande C, Maneiro I, Riveiro I, Barreiro A, Pazos Y (2002) Estimation of copepod trophic niche in the field using amino acids and marker pigments. Mar Ecol Prog Ser 239:147–156
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Sympos Ser 41:95–98
Harper GL, King RA, Dodd CS, Harwood JD, Glen DM, Bruford MW, Symondson WOC (2005) Rapid screening of invertebrate predators for multiple prey DNA targets. Mol Ecol 14:819–827
Harwood JD, Obrycki JJ (2005) Quantifying aphid predation rates of generalist predators in the field. Eur J Entomol 102:335–350
Hoogendoorn M, Heimpel GE (2001) PCR-based gut content analysis of insect predators: using ribosomal ITS-1 fragments from prey to estimate predation frequency. Mol Ecol 10:2059–2067
Houde SEL, Roman MR (1987) Effects of food quality on the functional ingestion response of the copepod Acartia tonsa. Mar Ecol Prog Ser 40:69–77
Irigoien X (1998) Gut clearance rate constant, temperature and initial gut contents: a review. J Plankton Res 20:997–1003
Irigoien X, Meyer B, Harris R, Harbour D (2004) Using HPLC pigment analysis to investigate phytoplankton taxonomy: the importance of knowing your species. Helgoland Mar Res 58:77–82
Jakobsen HH, Halvorsen E, Hansen BW, Visser AW (2005) Effects of prey motility and concentration on feeding in Acartia tonsa and Temora longicornis: the importance of feeding modes. J Plankton Res 27:775–785
Jansen S, Bathmann U (2007) Algae viability within copepod faecal pellets: evidence from microscopic examinations. Mar Ecol Prog Ser 337:145–153
Jarman SN, Gales NJ, Tierney M, Gill PC, Elliott NG (2002) A DNA-based method for identification of krill species and its application to analysing the diet of marine vertebrate predators. Mol Ecol 11:2679–2690
Jarman SN, Redd KS, Gales NJ (2006) Group-specific primers for amplifying DNA sequences that identify Amphipoda, Cephalopoda, Echinodermata, Gastropoda, Isopoda, Ostracoda and Thoracica. Mol Ecol Notes 6:268–271
Kaartvedt S, Larsen T, Hjelmseth K, Onsrud MSR (2002) Is the omnivorous krill Meganyctiphanes norvegica primarily a selectively feeding carnivore? Mar Ecol Prog Ser 228:193–204
Kanagawa T (2003) Bias and artifacts in multitemplate polymerase chain reactions (PCR). J Biosci Bioeng 96:317–323
Kleppel GS (1993) On the diets of calanoid copepods. Mar Ecol Prog Ser 99:183–195
Kleppel GS, Frazel D, Pieper RE, Holliday DV (1988) Natural diets of zooplankton off southern California. Mar Ecol Prog Ser 49:231–241
Landry MR, Fagerness VL (1988) Behavioral and morphological influences on predatory interactions among marine copepods. Bull Mar Sci 43:509–529
Levinsen H, Turner JT, Nielsen TG, Hansen BW (2000) On the trophic coupling between protists and copepods in arctic marine ecosystems. Mar Ecol Prog Ser 204:65–77
Liaud M-F, Brandt U, Scherzinger M, Cerff R (1997) Evolutionary origin of Cryptomonad microalgae: two novel chloroplast/cytosol-specific GAPDH genes as potential markers of ancestral endosymbiont and host cell components. J Mol Evol 44:S28–S37
Liu H, Dagg MJ, Strom S (2005) Grazing by the calanoid copepod Neocalanus cristatus on the microbial food web in the coastal Gulf of Alaska. J Plankton Res 27:647–662
Long JD, Hay ME (2006) When intraspecific exceeds interspecific variance: effects of phytoplankton morphology and growth phase on copepod feeding and fitness. Limnol Oceanogr 51:988–996
Lyons MM, Smolowitz R, Dungan CF, Roberts SB (2006) Development of a real time quantitative PCR assay for the hard clam pathogen Quahog Parasite Unknown (QPX). Dis Aquat Org 72:45–52
Marine Zooplankton Colloquium 2 (2001) Future marine zooplankton research: a perspective. Mar Ecol Prog Ser 222:297–308
Martin DL, Ross RM, Quetin LB, Murray AE (2006) Molecular approach (PCR-DGGE) to diet analysis in young Antarctic krill Euphausia superba. Mar Ecol Prog Ser 319:155–165
Mauchline J (1998) The biology of calanoid copepods. In: Blaxter JHS, Southward AJ, Tyler PA (eds) Advances in marine biology. Academic, San Diego, pp 710
McLeroy-Etheridge SL, McManus GB (1999) Food type and concentration affect chlorophyll and carotenoid destruction during copepod feeding. Limnol Oceanogr 44:2005–2011
Montresor M, Nuzzo L, Mazzocchi MG (2003) Viability of dinoflagellate cysts after the passage through the copepod gut. J Exp Mar Biol Ecol 287:209–221
Moorthi SD, Countway PD, Stauffer BA, Caron DA (2006) Use of quantitative real-time PCR to investigate the dynamics of the red tide dinoflagellate Lingulodinium polyedrum. Microb Ecol 52:136–150
Nejstgaard J, Tang K, Steinke M, Dutz J, Koski M, Antajan E, Long J (2007) Zooplankton grazing on Phaeocystis: a quantitative review and future challenges. Biogeochemistry 83:147–172
Nejstgaard JC, Båmstedt U, Bagøien E, Solberg PT (1995) Algal constraints on copepod grazing. Growth state, toxicity, cell size, and season as regulating factors. ICES J Mar Sci 52:347–357
Nejstgaard JC, Frischer ME, Raule CL, Gruebel R, Kohlberg KE, Verity PG (2003) Molecular detection of algal prey in copepod guts and faecal pellets. Limnol Oceanogr Meth 1:29–38
Nejstgaard JC, Gismervik I, Solberg PT (1997) Feeding and reproduction by Calanus finmarchicus, and microzooplankton grazing during mesocosm blooms of diatoms and the coccolithophore Emiliania huxleyi. Mar Ecol Prog Ser 147:197–217
Nejstgaard JC, Naustvoll L-J, Sazhin A (2001) Correcting for underestimation of microzooplankton grazing in bottle incubation experiments with mesozooplankton. Mar Ecol Prog Ser 221:59–75
Ohman MD (1992) Immunochemical recognition of oligotrich ciliates. Mar Biol 114:653–660
Ohman MD, Runge JA (1994) Sustained fecundity when phytoplankton resources are in short supply: omnivory by Calanus finmarchicus in the Gulf of St Lawrence. Limnol Oceanogr 39:21–36
Øresland V, Ward P (1993) Summer and winter diet of four carnivorous copepod species around South Georgia. Mar Ecol Prog Ser 98:1–2
Paffenhöfer G-A, Knowles SC (1980) Omnivorousness in marine planktonic copepods. J Plankton Res 2:355–365
Paffenhöfer G-A, Lewis KD (1990) Perceptive performance and feeding behavior of calanoid copepods. J Plankton Res 12:933–946
Pandolfini E, Thys I, Leporcq B, Descy J-P (2000) Grazing experiments with two freshwater zooplankters: fate of chlorophyll and carotenoid pigments. J Plankton Res 22:305–319
Pasternak A, Wassmann P, Riser CW (2005) Does mesozooplankton respond to episodic P inputs in the Eastern Mediterranean? Deep Sea Res Part II: Topical Stud Oceanogr 52:2975–2989
Peterson WT, Dam HG (1996) Pigment ingestion and egg production rates of the calanoid copepod Temora longicornis: implications for gut pigment loss and omnivorous feeding. J Plankton Res 18:855–861
Planque B, Hays GC, Ibanez F, Gamble JC (1997) Large scale spatial variations in the seasonal abundance of Calanus finmarchicus. Deep Sea Res Part I: Oceanogr Res Papers 44:315–326
Pond DW, Harris RP, Brownlee C (1995) A microinjection technique using a pH-sensitive dye to determine the gut pH of Calanus helgolandicus. Mar Biol 123:75–79
Prescott DM (1994) The DNA of ciliated protozoa. Microbiol Rev 58:233–267
Rosel PE, Kocher TD (2002) DNA-based identification of larval cod in stomach contents of predatory fishes. J Exp Mar Biol Ecol 267:75–88
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual. Harbor Laboratory Press, Cold Spring
Sell AF, van Keuren D, Madin LP (2001) Predation by omnivorous copepods on early developmental stages of Calanus finmarchicus and Pseudocalanus spp. Limnol Oceanogr 46:953–959
Sheppard SK, Harwood JD (2005) Advances in molecular predator–prey ecology. Funct Ecol 19:751–762
Stoecker DK, Capuzzo JM (1990) Predation on protozoa: its importance to zooplankton. J Plankton Res 12:891–908
Symondson WOC (2002) Molecular identification of prey in predator diets. Mol Ecol 11:627–641
Tande KS, Drobysheva S, Nesterova V, Nilssen EM, Edvardsen A, Tereschenko V (2000) Patterns in the variations of copepod spring and summer abundance in the northeastern Norwegian Sea and the Barents Sea in cold and warm years during the 1980s and 1990s. ICES J Mar Sci 57:1581–1591
Theilacker GH, Lo NCH, Townsend AW (1993) An immunochemical approach to quantifying predation by euphasiids on the early life stages of anchovy. Mar Ecol Prog Ser 92:35–50
Tourova TP (2003) Copy number of ribosomal operons in prokaryotes and its effect on phylogenetic analyses. Microbiology 72:389–402
Troedsson C, Frischer ME, Nejstgaard JC, Thompson EM (2007) Molecular quantification of differential ingestion and particle trapping rates by the appendicularian Oikopleura dioica as a function of prey size and shape. Limnnol Oceanogr 52:416–427
Turner JT, Tester PA, Hettler WF (1985) Zooplankton feeding ecology: a laboratory study of predation on fish eggs and larvae by the copepods Anomalocera ornata and Centropages typicus. Mar Biol 90:1–8
Venter JD, Van Wyngaardt S, Verschoor JA, Lipinski MR, Verheye HM (1999) Detection of zooplankton prey in squid paralarvae with immunoassay. J Immunoassay 20:127–149
Verity PG, Paffenhöfer G-A (1996) On assessment of prey ingestion by copepods. J Plankton Res 18:1767–1779
Verschoor AM, Boonstra H, Meijer T (2005) Application of stable isotope tracers to studies of zooplankton feeding, using the rotifer Brachionus calyciflorus as an example. Hydrobiologia 546:535–549
Vestheim H, Edvardsen B, Kaartvedt S (2005) Assessing feeding of a carnivorous copepod using species specific PCR. Mar Biol 147:381–385
Wolfe GV (2000) The chemical defense ecology of marine unicellular plankton: constraints, mechanisms, and impacts. Biol Bull 198:225–244
Wong ML, Medrano JF (2005) Real-time PCR for mRNA quantitation. BioTechniques 39:75–85
Woodson CB, Webster DR, Weissburg MJ, Yen J (2007) Cue hierarchy and foraging in calanoid copepods: ecological implications of oceanographic structure. Mar Ecol Prog Ser 330:163–177
Yen J (1985) Selective predation by the carnivorous marine copepod Euchaeta elongata: laboratory measurements of predation rates verified by field observations of temporal and spatial feeding patterns. Limnol Oceanogr 30:577–597
Zhu F, Massana R, Not F, Marie D, Vaulot D (2005) Mapping of picoeucaryotes in marine ecosystems with quantitative PCR of the 18S rRNA gene. FEMS Microbiol Ecol 52:79–92
Acknowledgments
We would like to thank the crews of the R.V. “Côtes de la Manche” for assistance, Valérie Gentilhomme as the coordinator of both PNEC & CPER programs, Alexei Sentchev for field equipment, Natacha Guiselin for her help with phytoplankton analysis of field samples, and Dr. Elvire Antajan for assistance with copepods during the field study. We also thank the five anonymous reviewers for their valuable suggestions. The figures were prepared by Ms. Anna Boyette (SkIO). This work was supported by the Norwegian Research Council (NRC) project 152714/120 30 to JCN, NRC project 145326/432 to CT, and the U.S. National Science Foundation Office of Polar Programs grant (OPP-00-83381) and the US Department of Energy Biotechnology Investigations—Ocean Margins Program (FG02-98EF 62531) to MEF.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S.D. Connell.
Rights and permissions
About this article
Cite this article
Nejstgaard, J.C., Frischer, M.E., Simonelli, P. et al. Quantitative PCR to estimate copepod feeding. Mar Biol 153, 565–577 (2008). https://doi.org/10.1007/s00227-007-0830-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0830-x