Abstract
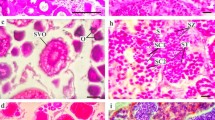
Spawning pattern (assessed by seasonal changes in ovarian developmental stages) and type of fecundity (assessed by analysis of oocyte-size frequency distributions) of the round herring Etrumeus teres were studied in relation to ovarian growth and seasonal changes in the gonadosomatic (GSI), hepatosomatic (HSI) and liposomatic (LSI) index as well as the somatic condition of spawners (CS) in a spawning ground of southern Japan. Except for summer, mature and recently spawned ovaries occurred all year round. Oogonia and primary oocytes were present in all ovaries, and cortical alveoli stage (CA) oocytes occurred in all mature, hydrated and partially spent (PS) females (PS: females containing post-ovulatory follicles). Before hydration, a clutch of larger yolked oocytes, undergoing synchronous growth (range 0.7–1.1 mm), was present in mature ovaries which was completely separated from a more heterogeneous clutch of oogonia, primary and secondary oocytes (<0.150 mm) and oocytes in the CA stage (range 0.15–0.60 mm). As vitellogenesis progressed, the yolked clutch increased in size but the CA oocytes remained arrested. The latter entered into the secondary growth phase when hydration started in the advanced batch. Ovarian growth was isometric in all developmental stages, validating the use of GSI, which showed a consistent monthly evolution among years. Spawning stopped in summer (July and August) and peaked in winter and spring. HSI correlated positively with GSI on both a monthly mean basis (r = 0.76) and individual fish basis (liver weight explained 67–83% of the variability in ovary weight when females were grouped into 1-unit GSI intervals) suggesting a significant role of liver in vitellogenesis. LSI and CS also showed marked seasonal changes peaking from summer to middle autumn. Overall results suggest that E. teres is a multiple spawner with a group-synchronous ovarian development and indeterminate annual fecundity, with the three processes linked to an isometric growth of the ovary. We propose that such a reproductive pattern is an adaptation to produce batches of large pelagic eggs through a protracted spawning season.








Similar content being viewed by others
References
Bagenal TB (1971) The interrelation of the size of fish eggs, the date of spawning and the production cycle. J Fish Biol 3:207–219
Barbieri-Lowerre SK, Chittenden ME Jr, Barbieri LR (1996) The multiple spawning pattern of weakfish in the Chesapeake Bay and Middle Atlantic Bight. J Fish Biol 48:1139–1163
Blaxter JHS, Hunter JR (1982) The biology of the clupeoid fishes. In: Blaxter JH, Russel FS, Yonge M (eds) Advances in marine biology, vol 30. Academic, Dublin, pp 1–223
Chullarson D, Mako H, Oka M, Matsuyama Y (1977) Studies on the fisheries biology of the round herring in the western Sea of Japan. Bull Sekai Reg Fish Res Lab 50:37–71
Claramunt G, Herrera G (1994) A new method to estimate the fraction of daily spawning females and the number of spawning in Sardinops sagax in northern Chile. Sci Mar 58:169–177
Claramunt G, Roa R (2001) An indirect approach to estimate spawning fraction as applied to Sardinops sagax from northern Chile. Sci Mar 65:87–94
Cone RS (1989) The need of reconsider the use of condition indices in fishery science. Trans Am Fish Soc 118:510–514
Craik JCA, Harvey SM (1987) The causes of buoyancy in eggs of marine teleosts. J Mar Biol Ass UK 67:169–182
Delahunty G, De Vlaming VL (1980) Seasonal relationships of ovary weight, liver weight and fat stores with body weight in the goldfish, Carrasius auratus (L.). J Fish Biol 16:5–13
DeMartini EE, Lau BB (1999) Morphometric criteria for estimating sexual maturity in two snappers, Etelis carbunculus and Pristipomoides sieboldii. Fish Bull 89:9–18
DeVlaming V, Grossman G, Chapman F (1982) On the use of gonadosomatic index. Comp Biochem Physiol A 73:31–39
Erickson DL, Hightower JE, Grossman GD (1985) The relative gonadal index. An alternative for quantification of reproductive condition. Comp Biochem Physiol A 81:117–120
Ganias K, Somarakis S, Machias A, Theodorou A (2003) Pattern of oocyte development and batch fecundity in the Mediterranean sardine. Fish Res 67:13–23
Hara I (1977) Population studies on round herring—I: on round herring fished off the western coast of San’in district. Bull Jpn Soc Sci Fish 43:265–270
Hayashi M, Taniguchi N, Yamaoka K (1988) Quantitative analysis on fish larvae and juveniles caught by sardine drag net in Tosa Bay, Japan. Rep Mar Biol Inst Kochi Univ 10:83–92
Hesp SA, Potter IC, Hall NG (2004) Reproductive biology and protandrous hermaphroditism in Acanthopagrus latus. Environ Biol Fish 70:257–272
Honda H, Hirota Y, Mitani T, Uehara S, Sakaji H, Nashida K (2002) Seasonal changes in length-frequency distributions and maturity conditions of round herring Etrumeus teres, spawning in Tosa Bay and the occurrence period of the young fish. Fish Biol Oceanogr Kur 3:75–83
Horwood JW, Walker MG (1990) Determinacy of fecundity in sole (Solea solea) from the Bristol Channel. J Mar Biol Assoc UK 70:803–813
Hunter JR, Goldberg SR (1980) Spawning incidence and batch fecundity in northern anchovy, Engraulis mordax. Fish Bull US 77:641–652
Hunter JR, Leong R (1981) The spawning energetics of female northern anchovy Engraulis mordax. Fish Bull US 79:215–230
Hunter JR, Macewicz BJ (1985) Measurement of spawning frequency in multiple spawning fishes. In: Lasker R (ed) An egg production method for estimating spawning biomass of pelagic fish: application to the northern anchovy, Engraulis mordax, vol 36. NOAA-NMFS, Tech Rep, pp 79–94
Hunter JR, Macewicz BJ, Lo NCH, Kimbrel CA (1992) Fecundity, spawning, and maturity of female Dover sole Microstomus pacificus, with an evaluation of assumptions and precision. Fish Bull 90:101–128
Isaac-Nahum VJ, Cardoso R del D, Servo G, Rosi-Wongtschowski CL del B (1988) Aspects of the spawning biology of the Brazilian sardine Sardinella brasiliensis (Steindachner, 1879), (Clupeidae). J Fish Biol 32:383–396
Ishida M, Mitani T, Uehara S, Honda H (2004) Stock assessment of the Pacific stock of the round herring Etrumeus teres. In: Stock assessment of the fisheries resources around Japan. Technical report. Fishery Agency and Fishery Research Agency, Tokyo, Japan, pp 471–481
Ichikawa T, Hirota Y (2004) Seasonal changes in primary productivity in Tosa Bay. Oceanogr Jpn 13:259–269
Kjesbu OS, Witthames PR, Solemdal P, Walker MG (1990) Ovulatory rhythm and a method to determine the stage of spawning in Atlantic Cod (Gadus morhua). Can J Fish Aquat Sci 47:1185–1193
Le Clus F (1979) Oocyte development and spawning frequency in the south west African pilchard Sardinops ocellata. Fish Bull S Afr 12:53–68
Lowerre-Barbieri SK, Chittenden ME, Barbieri LR (1996) The multispawning pattern of weakfish in the Chesapeake Bay and Middle Atlantic Bight. J Fish Biol 48:1139–1163
Luo J, Musick JA (1991) Reproductive biology of the bay anchovy in Chesapeake Bay. Trans Am Fish Soc 120:701–710
Maddock DM, Burton MPM (1998) Gross and histological observations of ovarian development and related condition changes in American plaice. J Fish Biol 53:928–944
Marino G, Azzuro E, Massari A, Finoia MG, Mandich A (2001) Reproduction in dusky grouper Epinephelus marginatus (Lowe 1834) from southern Mediterranean. J Fish Biol 58:909–927
Marza VD (1938) Histophysiologie de l’ovogenèse. Herman, Paris
Militelli MI, Macchi GJ (2004) Spawning and fecundity of king weakfish, Macrodon ancylodon, in the Rio de la Plata estuary, Argentina—Uruguay. J Mar Biol Assoc UK 84:443–447
Morimoto H (1998) Relationship between batch fecundity and egg size in Japanese sardine Sardinops melanostictus in Tosa Bay and off Kii Channel, southwestern Japan form 1990 to 1993. Fish Sci 64:220–227
Nichol GD, Acuna EI (2000) Annual and batch fecundity of the yellowfin sole, Limanda aspera, in the eastern Bering Sea. Fish Bull 99:108–122
Ohshimo S (2004) Stock assessment of the Pacific stock of the round herring Etrumeus teres. In: Stock assessment of the fisheries resources around Japan. Technical report. Fishery Agency and Fishery Research Agency. Tokyo, Japan, pp 482–497
Patterson KR (1992) An improved method for studying the condition of fish, with an example using Pacific sardine Sardinops sagax (Jenyns). J Fish Biol 40:821–831
Pina T, Esteves E, Andrade JP (2003) Gross and histological observation of ovarian development in twaite shad, Alosa fallax fallax, from the rivers Mira and Guadiana (Portugal). Sci Mar 67:313–322
Sokal RR, Rohlf FJ (1997) Biometry, 3rd edn. W. H. Freeman, San Francisco 887 p
Somarakis S, Ganias K, Tsepes G, Koutsikopoulos (2004) Ovarian allometry and the use of gonadosomatic index: a case of study in the Mediterranean sardine, Sardina pilchardus. Mar Biol 146:181–189
Somarakis S, Ganias K, Slapatis A, Koutsikopolus C, Machias S, Papaconstantinou C (2006) Spawning habitat and daily egg production of sardine (Sardina pilchardus) in the eastern Mediterranean. Fisher Ocenogr 15:281–292
Stequert B, Menard F, Marchal E (2003) Reproductive biology of Vinciguerria nimbaria in the equatorial waters of the eastern Atlantic Ocean. J Fish Biol 62:1116–1136
Taylor RG, Murphy M (1992) Reproductive biology of swordfish Xiphias gladius in the Straits of Florida and adjacent waters. Fish Bull US 90:809–816
Taylor RG, Grier HJ, Whittington JA (1998) Spawning rhythms of common snook in Florida. J Fish Biol 53:502–520
Tomasini JA, Collart D, Quignard JP (1999) Reserve management strategy for the sand smelt from brackish lagoons in southern France. J Mar Biol Assoc UK 79:145–151
Wallace RA, Selman K (1979) Physiological aspects of oogenesis in two species sticklebacks, Gasterosteus aculeatus L. and Apeltes quadracus (Mitchill). J Fish Biol 14:551–564
Wallace RA, Selman K (1981) Cellular and dynamic aspects of oocyte size in teleosts. Am Zool 21:325–343
Wright PJ (1992) Ovarian development, spawning frequency and batch fecundity in Encrasicholina heteroloba (Ruppell, 1858). J Fish Biol 40:833–844
Wyatt T (1980) The growth season in the sea. J Plank Res 2:81–91
Yamada H (1994) Ecology of round herring Etrumeus teres in Kumano-nada Sea. Bull Jpn Soc Fish Oceanogr 58:286–291
Yanagawa S (1996) Batch fecundity and spawning period of the round herring in Tosa Bay. In: Report of stock assessment of fishery resource by prefectures in southwestern Japan, vol 4, pp 43–53
Yoneda M, Tokimura M, Fujita H, Takeshita N, Takeshita K, Matsuyama M, Matsuura S (1998) Reproductive cycle and sexual maturity of the anglerfish Lophiomus setigerus in the East China Sea with a note on specialized spermatogenesis. J Fish Biol 53:164–178
Acknowledgments
This study was carried out while GP was supported by a postdoctoral fellowship from the Japan Society for the Promotion of Science (No. P 03522). We express our gratitude to the captain and crews of the research vessel Kotakamaru for their assistance in the field. Special thanks to Mrs. Kazu Sumikawa for assistance in Laboratory analysis and Joel O’Dea for providing constructive comments on an early draft of this MS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Nishida.
An erratum to this article is available at http://dx.doi.org/10.1007/s00227-007-0876-9.
Rights and permissions
About this article
Cite this article
Plaza, G., Sakaji, H., Honda, H. et al. Spawning pattern and type of fecundity in relation to ovarian allometry in the round herring Etrumeus teres . Mar Biol 152, 1051–1064 (2007). https://doi.org/10.1007/s00227-007-0756-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0756-3