Abstract
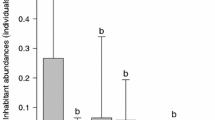
Delimiting communities in marine habitats is difficult because co-occurring species often have different life histories and the life stages experience the environment at different spatial scales. The habitat of a particular community is embedded within a larger habitat or ecosystem with many species shared between the focal community and the larger system. Pen shells (Atrina rigida) are large bivalves that, once the mollusk dies, provide shelter for motile species and hard substrate for settling larval invertebrates and egg-laying fishes. In St. Joseph’s Bay, Florida (29°45′N, 85°15′W), pen shells are the most abundant source of hard substrate, especially inside sea grass (Thalassia testudinum) beds, where they reach densities of 0.1–4.0 m−2. This study, which was conducted from May to August 2005, measured the overlap in species densities between dead pen shells and the surrounding sea grass communities at eight sites to determine the discreteness of the pen shell communities. Of the 70-epibenthic taxa recorded, 66% were found on the pen shells but not in the surrounding sea grass habitat. Community structure, which varied among shells within sites and among the eight sites, could be related to sea grass characteristics such as blade density and length either directly (e.g., inhabitants of pen shells directly benefit from the surrounding sea grass) or indirectly (e.g., pen shells and sea grass both benefit from similar factors such as current and nutrients). Pen shells were randomly distributed at several spatial scales within the 15 × 15 m sites as were many motile species. Two exceptions were the shrimp, Palaemon floridanus and the amphipod, Dulichella appendiculata, whose distributions were clumped. Most of the sessile species had clumped distributions, tending to be very abundant when they were present. These pen shell communities provide an opportunity for experimental studies of factors affecting species diversity on small, discrete, naturally occurring habitats.



Similar content being viewed by others
References
Bousfield EL (1973) Shallow-water Gammaridean Amphipoda of New England. Cornell University Press, Ithaca
Chase JM, Amarasekare P, Cottenie K, Gonzalez A, Holt RD, Holyoak M, Hoopes MF, Leibold MA, Loreau M, Mouquet N, Shurin JB, Tilman D (2005) Competing theories for competitive metacommunities. In: Holyoak M, Leibold MA, Holt RD (eds) Metacommunities. Spatial dynamics and ecological communities. University of Chicago Press, Chicago, pp 335–354
Cornell HV, Lawton JH (1992) Species interactions, local and regional processes, and limits to richness of ecological communities: a theoretical perspective. J Anim Ecol 61:1–12
Cummings VJ, Thrush SF, Hewitt JE, Turner SJ (1998) The influence of the pinnid bivalve Atrina zelandica (Gray) on benthic macroinvertebrate communities on soft-sediment habitats. J Exp Mar Biol Ecol 228:227–240
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Hewitt JE, Thrush SF, Legendre P, Cummings VJ, Norkko A (2002) Integrating heterogeneity across spatial scales: interactions between Atrina zelandica and benthic macrofauna. Mar Ecol Progr Ser 239:115–128
Holyoak M, Leibold MA, Holt RD (2005) Metacommunities. Spatial dynamics and ecological communities. University of Chicago Press, Chicago
Huston MA (1999) Local processes and regional patterns: appropriate scales for understanding variation in the diversity of plants and animals. Oikos 86:393–401
Jenkins SR (2005) Larval habitat selection, not larval supply, determines settlement patterns and adult distribution in two chthamalid barnacles. J Anim Ecol 74:893–904
Keough MJ (1984) Dynamics of the epifauna of the bivalve Pinna bicolor: interactions among recruitment, predation, and competition. Ecology 65:677–688
Koch EW, Gust G (1999) Water flow in the tide- and wave-dominated beds of the seagrass Thalassia testudinum. Mar Ecol Progr Ser 184:63–72
Krebs CJ (1999) Ecological methodology. Addison-Welsey Educational Publishers, Menlo Park
Kuhlmann ML (1996) Indirect effects among species in a Northern Gulf of Mexico seagrass community. PhD Dissertation, Department of Biological Science, Florida State University, Tallahassee
Mora C, Sale PF (2002) Are populations of coral reef fish open or closed? Trends Ecol Evol 17:422–428
Munguia P (2004) Successional patterns on pen shell communities at local and regional scales. J Anim Ecol 73:64–74
Munguia P (2006) Diversity patterns of pen shell (Atrina rigida) communities. PhD Dissertation. Department of Biological Science, Florida State University, Tallahassee
Norkko A, Hewitt JE, Thrush SF, Funnell GA (2006) Conditional outcomes of facilitation by a habitat-modifying subtidal bivalve. Ecology 87:226–234
Olson RR (1985) The consequences of short-distance larval dispersal in a sessile marine invertebrate. Ecology 66:30–39
Palmer MA, Allan JD, Butman CA (1996) Dispersal as a regional process affecting the local dynamics of marine and stream benthic invertebrates. Trends Ecol Evol 11:322–326
Quinn GP, Keough MJ (2003) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Ricklefs RE, Schluter D (1993) Species diversity in ecological communities. Chicago University Press, Chicago
Roughgarden J, Iwasa Y, Baxter C (1985) Demographic theory for an open marine population with space-limited recruitment. Ecology 66:54–67
Shmida A, Wilson MV (1985) Biological determinants of species diversity. J Biogeogr 12:1–20
Sotka EE, Hay ME (2002) Geographic variation among herbivore populations in tolerance for a chemically rich seaweed. Ecology 83:2721–2735
Srivastava DS, Kolasa J, Bengtsson J, Gonzalez A, Lawler SP, Miller T, Munguia P, Schneider D, Trzcinski MK (2004) Miniature worlds: are natural microcosms the new model systems for ecology? Trends Ecol Evol 19:379–384
Tilman D (1994) Competition and biodiversity in spatially structured habitats. Ecology 75:2–16
Warwick RM, McEvoy AJ, Thrush SF (1997) The influence of Atrina zelandica Gray on meiobenthic nematode diversity and community structure. J Exp Mar Biol Ecol 214:231–247
Wolfe SH, Reidenauer JA, Means DB (1988) An ecological characterization of the Florida panhandle. US Fish Wildl Service Biol Report 88:277
Acknowledgments
I would like to thank the indefatigable pen shell crew of ‘05, C. Stokes, B. Ehrmann, and A. Schweikart. C. terHorst provided assistance in the field and in the drawing room. D. Levitan and T. Miller provided insightful comments and support in the development of this study. B. Inouye and J. Wulff helped figure out statistical and ecological problems that kept surfacing from time to time. R. Karlson gave useful feedback to the final draft. I borrowed the invertebrate sweeping technique from E. Duffy, to whom I am grateful. The manuscript benefited from the comments and suggestions of three reviewers. I would like to give special thanks to J. Huffman, R. Ogles, M. Steele, and all the staff of the St. Joe Bay Buffer Preserve and Cape San Blas State Park. The research was partially supported by a Gramling Research Award, FSU.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle.
Electronic supplementary material
Below is the link to the electronic supplementary material.
227_2007_670_MOESM1_ESM.doc
APPENDIX 1. Species list of pen shell inhabitants ranked by abundance and ordered by (A)motile and (B) sessile species. Average pen shell abundance (% cover for sessile species) with standard deviations in parentheses. (DOC 53 kb)
Rights and permissions
About this article
Cite this article
Munguia, P. Spatial structure of communities on dead pen shells (Atrina rigida) in sea grass beds . Mar Biol 152, 149–156 (2007). https://doi.org/10.1007/s00227-007-0670-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0670-8