Abstract
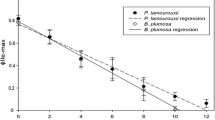
Previous studies on kleptoplasty in sacoglossans have used different methodology to investigate how long the sacoglossans are able to keep photosynthetically active (functional) chloroplasts. In this study we have used Pulse Amplitude Modulated Fluorometry to measure the quantum yield of charge separation in photosystem II in dark acclimated cells (ΦIIe) to detect the status of photosynthetic activity. Seven species of sacoglossa, Plakobranchus ocellatus, Elysia timida, Elysia sp, Elysia tomentosa, Thuridilla carlsoni, T. lineolata and Elysiella pusilla, were investigated regarding their ability to retain functional chloroplasts (RFC). The results show three different levels of RFC’s where P. ocellatus has the longest RFC for more than 11 months, E. timida with a RFC 1/4 than P. ocellatus (almost 3 months) and the rest with RFC’s down to 1/22 of P. ocellatus (up to 15 days). Based on these results, and compared to previous studies, eight different levels of retention abilities of non-functional and functional chloroplasts in sacoglossans are proposed. As far as we know, this is a novel method studying chloroplast functionality in sacoglossans.

Similar content being viewed by others
References
Berges JA, Charlebois DO, Mauzerall DC, Falkowski PG (1996) Differential effects of nitrogen limitation on photosynthetic efficiency on photosystems I and II in microalgae. Plant Physiol 110:689–696
Bjørkmann O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77k among vascular plants of diverse origins. Planta 170:489–504
Cha Y, Mauzerall DC (1992) Energy-storage of linear and cyclic electron flows in photosynthesis. Plant Physiol 100(4):1869–1877
Clark KB (1984) New records and synonymies of Bermuda opisthobranchs (Gastropoda). The Nautilus 98(2): 85–97
Clark KB, Busacca M (1978) Feeding specificity and chloroplast retention in four tropical ascoglossa, with a discussion of the extent of chloroplast symbiosis and the evolution of the order. J Mollusc Stud 44:272–282
Clark KB, Jensen KR, Stirts HM (1990) Survey for functional kleptoplasty among West Atlantic Ascoglossa (= Sacoglossa) (Mollusca: Opisthobrachia). Veliger 33(4):339–345
Demmig B, Bjørkman O (1987) Comparison of the effect of excessive light on chlorophyll fluoresence (77k) and photon yield of O-2 evolution in leaves of higher plants. Planta 171(2):171–184
Falkowski PG, Raven JA (1997) Aquatic photosynthesis. Blackwell, USA, p 375
Genty B, Briantis JM, Baker NR (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990:87–92
Govindjee (1995) Sixty-three years since Kautsky: chlorophyll a fluorescence. Aust J Plant Physiol 22:131–160
Grant BR, Borowitzka MA (1984) The chloroplasts of giant celled and coenocytic algae: biochemistry and structure. Bot Rev 50(3):267–307
Greene RW (1970) Symbiosis in Sacoglossan opisthobranchs: functional capacity of symbiotic chloroplasts. Mar Biol 7(2):138–142
Hawes CR (1979) Ultrastructural aspects of the symbiosis between algal chloroplasts and Elysia viridis. New Phytol 83(2):445
Hinde R (1980) Chloroplast “symbiosis” in sacoglossans molluscs. In: Schwemmler W, Schenk HEA (eds) Endocytobiology, endosymbiosis and cell biology. Proceedings of the international colloquium on endosymbiosis and cell research. Tübingen, Germany, Walter de Gruyter, pp 729–36
Hinde R, Smith DC (1972) Persistence of functional chloroplasts in Elysia viridis (Opisthobranchia, Sacoglossa). Nature 239:30–31
Hinde R, Smith DC (1974) ‘Chloroplast symbiosis’ and the extent to which it occurs in Sacoglossa (Gastropoda: Mollusca). Biol J Linn Soc 6(4):349–356
Hinde R, Smih DC (1975) The role of photosynthesis in the nutrition of the mollusc Elysia viridis. Biol J Linn Soc 7:161–171
Ireland C, Scheuer PJ (1979) Photosynthetic marine molluscs: in vivo 14C incorporation into metabolites of the sacoglossan Plakobranchus ocellatus. Science 205(4409):922–923
Jensen KR (1980) A review of sacoglossan diets, with comparative notes on radular and buccal anatomy. Mal Rev 15:55–77
Jensen KR (1992) Anatomy of some Indo-Pacific elysiidae (Opisthobranchia: Sacoglossa (= Ascoglossa)), with a discussion of the generic division and phylogeny. J Mollusc Stud 58:257–296
Jensen KR (1996) Phylogenetic systematics and classification of the Sacoglossa (Mollusca, Gastropoda, Opisthobranchia). Philos Trans R Soc Lond B 351:91–122
Jensen KR (1997) Evolution of the sacoglossa (Mollusca, Opisthobranchia) and the ecological associations with their food plants. Evol Ecol 11:301–335
Kolber Z, Falkowski PG (1993) Use of active fluorescence to estimate phytoplankton photosynthesis in situ. Limnol Oceanogr 38(8):1646–1665
van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth Res 25:147–150
Kromkamp JC, Forster RM (2003) Use of variable fluorescence measurements in aquatic ecosystems: differences between multiple and single turnover measuring protocols and suggested terminology. Eur J Phycol 28(2):103–112
Marin A, Ros JD (1988) The Sacoglossa (Mollusca, Opisthobranchia) of South East Iberian peninsula. A catalogue of species and presence of algal chloroplasts in them. Iberius 8(1):25–49
Marin A, Ros JD (1992) Dynamics of a peculiar plant–herbivore relationship: the photosynthetic ascoglossan Elysia timida and the chlorophycean Acetabularia acetabulum. Mar Biol 112:677–682
Rumpho ME, Summer EJ, Manhart JR (2000) Solar-powered sea slugs. Mollusc/algal chloroplast symbiosis. Plant Physiol 123:29–38
Taylor DL (1968) Chloroplasts as symbiotic organelles in the digestive gland of Elysia viridis (Gastropoda: Opisthobranchia). J Mar Biol Assoc UK 48:1–15
Trench RK (1969) Chloroplasts as functional endosymbionts in the mollusc Tridachia crispata (Bergh), (Opisthobranchia, Sacoglossa). Nature 22:1071–1072
Trench RK (1975) Of ‘leaves that crawl’. Functional chloroplasts in animal cells. Symp Soc Exp Biol 29:229–265
Trench RK, Smith DC (1970) Synthesis of pigment in symbiotic chloroplasts. Nature 227:196–197
Trench RK, Boyle JE, Smith DC (1973) The association between chloroplasts of Codium fragile and the mollusc Elysia viridis. II. Chloroplast ultrastructure and photosynthetic carbon fixation in E. viridis. Proc R Soc Lond B 184:63–81
Trench RK, Olhorst S (1976) The stability of chloroplasts from siphonaceous algae in symbiosis with sacoglossan mollusca. New Phytol 76:99–109
Wägele H, Johnsen G (2001) Observations on the histology and photosynthetic performance of “solar powered” opisthobranchs (Mollusca, Gastropoda, Opisthobranchia) containing symbiotic chloroplasts or zooxanthellae. Org Divers Evol 1:193–210
Waugh GR, Clark KB (1986) Seasonal and geographic variation in chlorophyll level of Elysia tuca (Ascoglossa: Opisthobranchia). Mar Biol 92:483–487
Williams SI, Walker DI (1999) Mesoherbivore–macroalgal interactions: feeding ecology of sacoglossan sea slugs (Mollusca, Opisthobranchia) and their effects on their food algae. Oceanogr Mar Biol Annu Rev 37:87–138
Acknowledgments
We would like to thank colleagues and staff at Lizard Island Research Station (Australia), and at the Faculty of Fisheries & Marine Science at the Sam Ratulangi University Manado (Sulawesi, Indonesia). This study was supported by the German Science Foundation to I. Burghardt and H. Wägele (SSP 1127: Wa 618/8) and by “The Improving Human Potential – Transnational Access to Research Infrastructures Programme of the European Commission” in Trondheim, Norway, and by the Norwegian Research Council to J. Evertsen (NFR 153790/120). Experiments comply with the current laws of the countries in which the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kühl.
Rights and permissions
About this article
Cite this article
Evertsen, J., Burghardt, I., Johnsen, G. et al. Retention of functional chloroplasts in some sacoglossans from the Indo-Pacific and Mediterranean. Mar Biol 151, 2159–2166 (2007). https://doi.org/10.1007/s00227-007-0648-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0648-6