Abstract
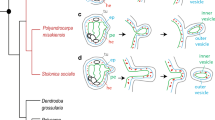
Asexual reproduction by external budding in Homoscleromorpha is reported for the first time. Two Mediterranean sponge species were studied, Oscarella lobularis and O. tuberculata. Buds are formed in the marginal basal part of sponge. Budding takes from 1 to 4 days and is defined in three budding stages. First, small irregular protuberances, consisting of external parental tissue, are formed. Second, they elongate and acquire more regular, nipple-like shape. These protuberances are tube like, their internal cavity derived from parental exhalant canal. The wall consists of three layers: (a) external layer is flagellated exopinacoderm, (b) internal one is flagellated endopinacoderm and (c) intermediate one is a thin layer of mesohyl. Third, a spherical bud with a large central cavity is formed. During budding, we did not observe cell proliferation or transdifferentiation either in budding zone or in any special mitotically active region. The bud attached to the substrate is similar to the rhagon developing after larva metamorphosis, it has a syconoid organization. Morphogenetically, budding in Oscarella differes from that in other sponges. Occurring by epithelial morphogenesis, it is similar to morphallaxis during regeneration. The presence in Homoscleromorpha of an epithelial morphogenesis is unique among sponges. This feature is shared by Homoscleromorpha and Eumetazoa.






Similar content being viewed by others
References
Battershill CN, Bergquist PR (1990) The influence of storms on asexual reproduction, recruitment, and survivorship of sponges. In: Rützler K (ed) New perspectives in sponge biology. Smithsonian, Washington, pp 397–403
Bond C (1992) Continuous cell movements rearrange anatomical structures in intact sponges. J Exp Zool 263:284–302
Bond C, Harris AK (1988) Locomotion of sponges and its physical mechanism. J Exp Zool 246:271–284
Borchiellini C, Manuel M, Alivon E, Boury-Esnault N, Vacelet J, Le Parco Y (2001) Sponge paraphyly and the origin of Metazoa. J Evol Biol 14:171–179
Borchiellini C, Chombard C, Manuel M, Alivon E, Boury-Esnault N, Vacelet J (2004) Molecular phylogeny of Demospongiae: implications for classification and scenarios of character evolution. Mol Phylogenet Evol 32:823–837
Boury-Esnault N (1970) Un phénomène de bourgeonnement externe chez l’éponge Axinella damicornis (Esper). Cah Biol Mar 11:491–496
Boury-Esnault N, De Vos L, Donadey C, Vacelet J (1984) Comparative study of the choanosome of Porifera: I. The Homoscleromorpha. J Morphol 180:3–17
Boury-Esnault N, Muricy G, Gallissian M-F, Vacelet J (1995) Sponges without skeleton: a new Mediterranean genus of Homoscleromorpha (Porifera, Demospongiae). Ophelia 43:25–43
Boury-Esnault N, Ereskovsky AV, Bézac C, Tokina D (2003) Larval development in Homoscleromorpha (Porifera, Demospongiae) first evidence of basal membrane in sponge larvae. Invertebr Biol 122:187–202
Boury-Esnault N, Solé-Cava AM, Thorpe JP, Boury-Esnault N (1992) Genetic and cytological divergence between colour morphs of the Mediterranean sponge Oscarella lobularis Schmidt (Porifera, Demospongiae, Oscarellidae). J Nat Hist 26:271–284
Boute N, Exposito JY, Boury-Esnault N, Vacelet J, Noro N, Miyazaki K, Yoshigato K, Garrone R (1996) Type IV collagen in sponges, the missing link in basement membrane ubiquity. Biol Cell 88:37–44
Brien P (1973) Les Démosponges. Morphologie et reproduction. In: Grassé PP (ed) Spongiaires 3(1). Masson, Paris, pp 133–461
Chen YH, Chen C-P, Chang K-H (1997) Budding cycle and bud morphology of the globe-shaped sponge Cinachyra australiensis. Zool Stud 36:194–200
Connes R (1964) Contribution a l’étude de la proliferation par voie asexuee chez le Sycon. Bull Soc Zool France 89:188–195
Connes R (1967) Structure et developpement des bourgeons chez l’éponge siliceuse Tethya lyncurium Lamarck. Recherches experimentales et cytologiques. Arch Zool Exp Générale 108:157–195
Connes R (1977) Contribution a l’etude de la gemmulogenese chez la démosponge marine Suberites domuncula (Olivi) Nardo. Arch Zool Exp Générale 118:391–407
Connes R, Paris J, Carriére D (1978) Etude du d‚veloppement des gemmules chez la démosponge marine Suberites domuncula (Olivi) Nardo. Ann Sci Nat Biol Anim 20:357–387
Corriero G, Liaci LS, Marzano CN, Gaino E (1998) Reproductive strategies of Mycale contarenii (Porifera, Demospongiae). Mar Biol 131:319–327
De Vos C (1965) Le bourgeonnement externe de l’éponge Mycale contarenii (Martens) (Demosponges). Bull Mus Nat Hist Natur Paris 37:548–555
Ereskovsky AV (2004) Comparative embryology of Sponges and its application for poriferan phylogeny. Boll Mus Ist Biol Univ Genova 68:301–318
Ereskovsky AV (2005) Comparative embryology of Sponges (Porifera). Sanct-Petersburg University Press, St. Petersburg
Ereskovsky AV, Boury-Esnault N (2002) Cleavage pattern in Oscarella species (Porifera, Demospongiae, Homoscleromorpha), transmission of maternal cells and symbiotic bacteria. J Nat Hist 36:1761–1775
Ereskovsky AV, Tokina DB, Bézac C, Boury-Esnault N. Metamorphosis of cinctoblastula larvae (Homoscleromorpha, Porifera). J Morphol (in press)
Exposito J-Y, Clusel C, Garrone R, Lethias C (2002) Evolution of Collagens. Anat Rec 268:302–316
Fell PE (1974) Porifera. In: Giese AC, Pearce JS (eds) Reproduction of marine invertebrates. Academic, New York, pp 51–132
Fell PE (1993) Porifera. In: Adiyodi KG, Adiyodi RG (eds) Reproductive biology of invertebrates. 6A: asexual propagation and reproductive strategies. Oxford and IBH, New Delhi, pp 1–44
Gaino E, Burlando B, Buffa P (1987) Structural and ultrastructural aspects of growth in Oscarella lobularis (Porifera, Demospongiae). Growth 51:451–460
Gaino E, Scalera Liaci L, Sciscioli M, Corriero G (2006) Investigation of the budding process in Tethya citrina and Tethya aurantium (Porifera, Demospongiae). Zoomorphology 125:87–97
Gilbert SF (2000) Developmental biology. Sinauer Associates, Inc. Sunderland
Hartman WD (1958) Natural history of the marine sponges of southern New England. Bull Peabody Mus Nat Hist Yale Univ 12:1–155
Herlant-Meewis H (1948) La gemmulation chez Suberites domuncula (Olivi) Nardo. Arch Anat Microsc Morphol Exp 37:289–322
Hoffman DK, Honegger TG (1990) Bud formation and metamorphosis in Cassiopea andromeda (Cnidaria: Scyphozoa): a developmental and ultrastructureal study. Mar Biol 105:509–518
Humbert-David N, Garrone R (1993) A six-armed, tenascin-like protein extracted from the Porifera Oscarella tuberculata (Homosclerophorida). Eur J Biochem 216:255–260
Ivanova-Kazas OM (1977) Asexual reproduction in animals. Leningrad University Press, Leningrad
Johnson MF (1978) Studies on the reproductive cycles of the calcareous sponges Clathrina coriacea and C. blanca. Mar Biol 50:73–79
Kawamura K, Fujiwara S (1995) Cellular and molecular characterization of transdifferentiation in the process of morphallaxis of budding tunicates. Semin. Cell Biol 6:117–126
Keller R, Lance A, Davidson D, Shook R (2003) How we are shaped: the biomechanics of gastrulation. Differentiation 71:171–205
Korotkova GP (1997) Regeneration in animals. Saint-Petersburg University Press, Saint-Petersburg
Li C-W, Chen J-Y, Hua T-E (1998) Precambrian sponges with cellular structures. Science 279:879–882
Manconi R, Pronzato R (2002) Suborder Spongillina subord. nov.: freshwater Sponges. In: Hooper JNA, Van Soest RWM (eds) Systema Porifera: a guide to the classification of sponges. Kluwer/Plenum, New York, pp 921–1020
Merejkowsky CD (1879) Reproduction des éponges par bourgeonnement extérieur. Arch Zool Expér Génér 8:417–433
Morgan TH (1901) Regeneration. MacMillan Company, London
Morris PJ (1993) The developmental role of the extracellular-matrix suggests a monophyletic origin of the kingdom animalia. Evolution 47:152–165
Müller WEG, Müller IM (2003) The hypothetical ancestral animal, the Urmetazoa: telomerase activity in sponges (Porifera). Serb Chem Soc 68:257–268
Muricy G, Boury-Esnault N, Bézac C, Vacelet J (1996) Cytological evidence for cryptic speciation in Mediterranean Oscarella species (Porifera, Homoscleromorpha). Can J Zool 74:881–896
Muricy G, Bézac C, Gallissian M-F, Boury-Esnault N (1999) Anatomy, cytology and symbiotic bacteria of four Mediterranean species of Plakina Schulze, 1880 (Demospongiae, Homosclerophorida). J Nat Hist 33:159–176
Nichols SA (2005) An evaluation of support for order-level monophyly and interrelationships within the class Demospongiae using partial data from the large subunit rDNA and cytochrome oxidase subunit I. Mol Phylog Evol 34:81–96
Otto JJ, Campbell RD (1977) Budding in Hydra attenuata: bud stages and fate map. J Exp Zool 200:417–223
Plotkin AS, Ereskovsky AV (1997) Ecological features of asexual reproduction of the White Sea sponge Polymastia mamillaris (Demospongiae, Tetractinomorpha) in Kandalaksha Bay. In: Ereskovsky AV, Keupp H, Kohring R (eds) Modern Problems of Poriferan Biology. Selb Fach Geowiss E20, Berlin pp 127–132
Saller U (1990) Formation and construction of asexual buds of the freshwater sponge Radiospongilla cerebellata (Porifera, Spongillidae). Zoomorphology 109:295–301
Schulze FE (1887) Report on the Hexactinellida collected by H.M.S. “Challenger” during the years 1873–1876. Zoology 21:1–514
Simpson TL (1984) The cell biology of sponges. Springer, Berlin Heidelberg New York
Topsent ME (1888) Sur les gemmules de quelques silicisponges marines. C R Acad Sci Paris 106:1298–1300
Acknowledgments
The authors thank Nicole Boury-Esnault and Jean Vacelet for helpful discussions Thierry Perez, Christian Marschal and Roland Graille for diving assistance (Centre d’Océanologie de Marseille, France). This work was funded by the programme RFBR N 06-04-48660 and Alexander Ereskovsky has received a special grant from the French “Ministère de l’Education nationale et de la Recherche” and a position of Invited Professor from the “Université de la Méditerranée”.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe.
Rights and permissions
About this article
Cite this article
Ereskovsky, A.V., Tokina, D.B. Asexual reproduction in homoscleromorph sponges (Porifera; Homoscleromorpha). Mar Biol 151, 425–434 (2007). https://doi.org/10.1007/s00227-006-0439-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0439-5