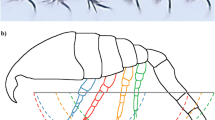
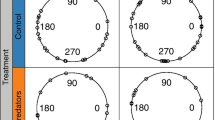
Abstract
Calanoid copepods typically exhibit escape reactions to hydrodynamic stimuli such as those generated by the approach of a predator. During the summers of 2000, 2001 and 2004, two small calanoid species, Temora turbinata Dana, 1849 and Paracalanus parvus Claus, 1863 were exposed to a visual predatory fish, the blenny Acanthemblemaria spinosa Metzelaar, 1919, and their predator–prey interactions were recorded using both high-speed and standard videographic techniques. Copepod escape reaction components, including swimming pattern, reactive distance, turning rate, and jump kinetics, were quantified from individual predation events using motion analysis techniques. Among the observed escape reaction components, differences were noted between the species’ swimming patterns prior to attack and their response latencies. Temora turbinata was a continuous cruiser and P. parvus exhibited a hop-and-sink swimming pattern. During periods of sinking, P. parvus stopped beating its appendages, which presumably reduced any self-generated hydrodynamic signals and increased perceptual abilities to detect an approaching predator. Response latency was determined for each copepod species using a hydrodynamic stimulus produced by a 1 ms acoustic signal. Response latencies of T. turbinata were significantly longer than those of P. parvus. Despite some apparent perceptual advantages of P. parvus, the blenny successfully captured both species by modifying its attack behavior for the targeted prey.







Similar content being viewed by others
References
Bainbridge R (1952) Underwater observations on the swimming of marine zooplankton. J Mar Biol Ass U K 31:107–112
Bundy MH, Paffenhöfer GA (1996) Analysis of flow fields associated with freely swimming calanoid copepods. Mar Ecol Prog Ser 133:99–113
Buskey EJ, Hartline DK (2003) High speed video analysis of the escape responses of the copepod Acartia tonsa to shadows. Bio Bull 204:28–37
Buskey EJ, Coulter C, Strom S (1993) Locomotory patterns of microzooplankton: potential effects on food selectivity of larval fish. Bull Mar Sci 53:29–43
Buskey EJ, Lenz PH, Hartline DK (2002) Escape behavior of planktonic copepods in response to hydrodynamic disturbances: high speed video analysis. Mar Ecol Prog Ser 235:135–146
Clarke RD (1992) Effects of microhabitat and metabolic rate on food intake, growth and fecundity of two competing coral reef fishes. Coral Reefs 1:199–205
Clarke RD, Buskey EJ, Marsden KC (2005) Effects of water motion and prey behavior on zooplankton capture by two coral reef fishes. Mar Biol 146:1145–1155
Fields DM, Yen J (1997) The escape behavior of marine copepods in response to a quantifiable fluid disturbance. J Plankton Res 19:1289–1304
Holling CS (1959) The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can Entomol 91:293–320
Kotrschal K, Lindquist DG (1986) The feeding apparatus of four Pacific tube blennies (Teleostei: Chaenopsidae): lack of ecomorphological divergence in syntopic species. Mar Ecol 7:241–253
Kramer DL, McLaughlin RL (2001) The behavioral ecology of intermittent locomotion. Am Zool 41:137–153
Lenz PH, Hartline DK (1999) Reaction times and force production during escape behavior of a calanoid copepod Undinula vulgaris. Mar Bio 133:249–258
Lenz PH, Hartline DK, Davis A (2000) The need for speed. I. Fast reactions and myelinated axons in copepods. J Comp Physiol A Sens Neural Behav Physiol 186:337–345
O’Brien WJ, Evans BI, Browman HI (1989) Flexible search tactics and efficient foraging in saltatory searching animals. Oecologia 80:100–110
Peterson WT, Ausubel SJ (1984) Diets and selective feeding by larvae of Atlantic mackerel Scomber scombrus on zooplankton. Mar Ecol Prog Ser 17:65–75
Ritchie JM (1984) Physiological basis of conduction in myelinated nerve fibers. In: Morrell P (ed) Myelin. Plenum Press, New York, pp 117–145
Suchman C (2000) Escape behavior of Acartia hudsonica copepods during interactions with scyphomedusae. J Plankton Res 22:2307–2323
Viitasalo M, Kiørboe T, Flinkman J, Pedersen L, Visser A (1998) Predation vulnerability of planktonic copepods: consequences of predator foraging strategies and prey sensory abilities. Mar Ecol Prog Ser 175:129–142
Wright DI, O’Brien WJ (1982) Differential location of Chaoborus larvae and Daphnia by fish: the importance of motion and visible size. Am Midl Nat 108:68–73
Yen J (2000) Life in transition: balancing inertial and viscous forces by planktonic copepods. Bio Bull 198:213–224
Zaret TM (1980) The effect of prey motion on planktivore choice. In: Kerfoot WC (ed) Evolution and ecology of zooplankton communities. University Press of New England, Hanover, pp 594–603
Acknowledgments
Funding for this research was provided by the National Science Foundation through Grant OCE-9910608 and The University of Texas Marine Science Institute Lund Fellowship. We thank R.D. Clarke for collecting the blennies and his assistance in their care and maintenance. We are grateful to P.H. Lenz and D.K. Hartline who shared both their advice and knowledge regarding sensory abilities of calanoid copepods. Technical assistance was provided by C. Hyatt. Guidelines of The University of Texas Institutional Animal Care and Use Committee were followed in the use of the blennies. This is The University of Texas Marine Science Institute contribution number 1418.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Waggett, R.J., Buskey, E.J. Calanoid copepod escape behavior in response to a visual predator. Mar Biol 150, 599–607 (2007). https://doi.org/10.1007/s00227-006-0384-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0384-3