Abstract
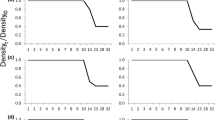
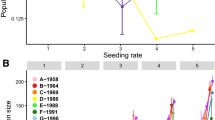
Sex change in many hermaphrodite animals has been suggested to be environmentally determined, especially socially. To investigate whether sex change in the protandric simultaneous hermaphrodite shrimp Lysmata wurdemanni (Gibbes 1850) is socially mediated, two experiments were conducted in the laboratory between September 2002 and April 2004 using laboratory-cultured shrimp that originated from Port Aransas, Texas, USA. The size at sex change (from male to simultaneous hermaphrodite) in this shrimp is variable, with the minimum around 2.4 cm in total length (TL). Large shrimp (2.4–4.5 cm TL) still in the male-phase (MP) have been found in the wild and laboratory environments. This study tested the hypothesis that large MP shrimp delay changing to the euhermaphrodite-phase (EP) due to social control. In the first experiment, ten shrimp were raised in large (110-l) and small (20-l) containers to test the effect of habitat size/density on sex change. The percentage of shrimp changing to EP was significantly higher in the large container (low density) than in the small container after 60 and 120 days. But after 570 days sex ratios were the same, 2 MP:8 EP. In the second experiment, group composition was changed over time to simulate population recruitment and mortality. MP shrimp delayed sex change when EP shrimp were present. However, if group structure is stable, some MP shrimp may not change sex during their lifetime. Under certain demographic conditions, such as when postlarvae (PL) were added to (simulating recruitment) or EP shrimp were removed from (simulating mortality) a group, all old MP (from original PL) shrimp changed to EP. The response of old MP shrimp to simulated recruitment is faster than to simulated mortality. The present study confirms that social control affects the size and timing of sex change in L. wurdemanni. However, some MP shrimp never change sex suggesting that genetics might also play a role in the sex ratios of L. wurdemanni populations.

Similar content being viewed by others
References
Allsop DJ, West SA (2003) Changing sex at the same relative body size. Nature 425:783–784
Baeza JA, Bauer RT (2004) Experimental test of socially mediated sex change in a protandric simultaneous hermaphrodite, the marine shrimp Lysmata wurdemanni (Caridea: Hippolytidae). Behav Ecol Sociobiol 55:544–550
Baldwin AP, Bauer RT (2003) Growth, survivorship, life span, and sex change in the hermaphroditic shrimp Lysmata wurdemanni (Decapoda: Caridea: Hippolytidae). Mar Biol 143:157–166
Bauer RT (2000) Simultaneous hermaphroditism in caridean shrimps: a unique and puzzling sexual system in the Decapoda. J Crust Biol 20:116–128
Bauer RT (2002a) Tests of hypotheses on the adaptive value of an extended male phase in the hermaphroditic shrimp Lysmata wurdemanni (Caridea: Hippolytidae). Biol Bull 203:347–357
Bauer RT (2002b) Reproductive ecology of a protandric simultaneous hermaphrodite, the shrimp, Lysmata wurdemanni (Decapoda: Caridea: Hippolytidae). J Crust Biol 22:742–749
Bauer RT, Holt GJ (1998) Simultaneous hermaphroditism in the marine shrimp Lysmata wurdemanni (Caridea: Hippolytidae): an undescribed sexual system in the decapod Crustacea. Mar Biol 132:223–235
Bauer RT, Newman WA (2004) Protandric simultaneous hermaphroditism in the marine shrimp Lysmata californica (Caridea: Hippolytidae). J Crust Biol 24:131–139
Bergström BI (1997) Do protandric pandalid shrimp have environmental sex determination? Mar Biol 128:397–407
Bergström BI (2000) The biology of Pandalus. Adv Mar Biol 38:1–245
Calado R, Narciso L, Morais S, Rhyne AL, Lin J (2003) A rearing system for the culture of ornamental decapod crustacean larvae. Aquaculture 218:329–339
Carpenter A (1978) Protandry in the freshwater shrimp, Paratya curvirostris (Heller, 1862) (Decapoda: Atyidae), with a review of the phenomenon and its significance in the Decapoda. J R Soc N Z 3:343–358
Charnov EL (1981) Sex reversal in Pandalus borealis: effect of a fishery? Mar Biol Lett 2:53–57
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton
Charnov EL, Anderson P J (1989) Sex change and population fluctuation in pandalid shrimp. Am Nat 134:824–827
Charnov EL, Bull J (1977) When is sex environmentally determined? Science 266:828–832
Charnov EL, Gotshall EW, Robinson JG (1978) Sex ratio: adaptive response to population fluctuations in pandalid shrimp. Science 200:204–206
Cole KS, Shapiro DY (1995) Social facilitation and sensory mediation of adult sex change in a cryptic, benthic marine goby. J Exp Mar Biol Ecol 186:65–75
d’Udekem d’Acoz C (2003) Lysmata seticaudata (Risso, 1816) and L. nilita Dohrn & Holthius, 1950 are protandrous simultaneous hermaphrodites (Decapoda, Caridea, Hippolytidae). Crustaceana 75:1149–1152
Fricke H, Fricke S (1977) Monogamy and sex change by aggressive dominance in coral reef fish. Nature 266:830–832
Ghiselin MT (1969) The evolution of hermaphroditism among animals. Q Rev Biol 44:189–208
Hattori A (1991) Socially controlled growth and size-dependent sex change in the anemonefish Amphiprion frenatus in Okinawa, Japan. Jpn J Ichthyol 38:165–177
Koeller P, Mohn R, Etter M (2000) Density dependent sex change in northern shrimp, Pandalus borealis, on the Scotian shelf. J Northwest Atl Fish 27:107–118
Lin J, Zhang D (2001) Reproduction in a simultaneous hermaphroditic shrimp, Lysmata wurdemanni: any two will do? Mar Biol 139:919–922
Little EE (1975) Chemical communication in maternal behaviour of crayfish. Nature 255:400–401
Marliave JB, Gergits WF, Aota S (1993) F10 pandalid shrimp: sex determination; DNA and dopamine as indicators of domestication; and outcrossing for wild pigment pattern. Zoo Biol 12:435–451
Muñoz RC, Warner RR (2003) Alternative contexts of sex change with social control in the bucktooth parrotfish, Sparisoma radians. Environ Biol Fishes 68:307–319
Policansky D (1982) Sex change in plants and animals. Annu Rev Ecol Syst 13:471–495
Ross RM (1990) The evolution of sex-change mechanism in fishes. Environ Biol Fishes 29:81–93
Sagi A, Snir E, Khalaila I (1997) Sexual differentiation in decapod crustaceans: role of the androgenic gland. Invertebr Reprod Dev 31:55–61
Shapiro DY (1987) Differentiation and evolution of sex change in fishes. BioScience 37:490–496
Sokal RR, Rohlf FJ (1995) Biometry. W. H. Freeman and company, New York
Suzuki K (1999) Androgenic gland hormone is a sex-reversing factor but cannot be a sex-determining factor in the female crustacean isopods Armadillidium vulgare. Gen Comp Endocrinol 115:370–378
Warner RR (1988) Sex change and the size advantage model. Trends Ecol Evol 3:133–136
Warner RR, Swearer SE (1991) Social control of sex change in the bluehead wrasse, Thalassoma bifasciatum (Pisces: Labridae). Biol Bull 181:199–204
Warner RR, Roberston DR, Leigh EG Jr (1975) Sex change and sexual selection. Science 190:633–638
Warner RR, Fitch DL, Standish JD (1996) Social control of sex change in the shelf limpet, Crepidula norrisiarum: size-specific responses to local group composition. J Exp Mar Ecol 204:155–167
Williams AB (1984) Shrimps, lobsters, and crabs of the Atlantic coast of the eastern United States, Maine to Florida. Smithsonian Institution Press, Washington
Zhang D, Lin J (2005a) Development of sexual morphs in two simultaneous hermaphroditic shrimp, Lysmata rathbunae and L. wurdemanni. Invertebr Reprod Dev 47:11–17
Zhang D, Lin J (2005b) Comparative mating success of smaller male-phase and larger male-role euhermaphrodite-phase shrimp, Lysmata wurdemanni (Caridea: Hippolytidae). Mar Biol 147:1387–1392
Zhang D, Lin J, Creswell RL (1998) Effects of the food and temperature on survival and development in the peppermint shrimp Lysmata wurdemanni. J World Aquac 29:471–476
Zupo V (2001) Influence of diet on sex determination of Hippolyte inermis Leach (Decapoda: Natantia) in the field. Hydrobiologia 449:131–140
Acknowledgments
We thank Andy Rhyne for raising the shrimp larvae. Drs. Bo Bergström, Martin Thiel, and anonymous reviewers provided valuable comments on an earlier draft. This study was supported by contracts from Shrimp Culture Technologies, Inc. and Sea Ag, Inc. The experiments comply with current laws of the United States.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Zhang, D., Lin, J. Effects of density and simulated recruitment and mortality on sex change in a protandric simultaneous hermaphroditic shrimp, Lysmata wurdemanni . Mar Biol 150, 639–645 (2007). https://doi.org/10.1007/s00227-006-0381-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0381-6