Abstract
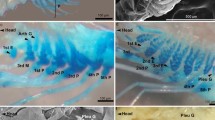
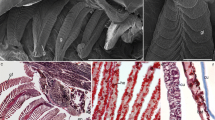
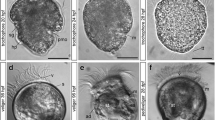
The development of gill chloride cells was examined in premetamorphic larvae (leptocephali) and juveniles (glass eels) of the Japanese eel, Anguilla japonica. Branchial chloride cells were detected by immunocytochemistry using an antiserum specific for Na+,K+-ATPase. The specificity and availability of the antiserum for the detection of Japanese eel chloride cells were confirmed by Western blot analysis. The chloride cells first appeared on the developing gill filaments in a mid larval stage of leptocephalus (32.2 mm). Both immunoreactivity and the number of chloride cells gradually increased as the fish grew to a late stage of leptocephalus over 54 mm. In glass eels just after metamorphosis, gill lamellae developed from the gill filaments, and a rich population of chloride cells was observed in the gill filaments. In glass eels collected at a coastal area, chloride cells were extensively distributed in the gill filaments. The chloride cell size decreased progressively in glass eels transferred from seawater (SW) to freshwater (FW), whereas there was no difference in cell number. In contrast, some Na+,K+-ATPase immunoreaction distinct from typical chloride cells was observed in the gill lamellae throughout FW-transferred fish, but disappeared in control fish maintained in SW for 14 days. These findings indicate that the gill and gill chloride cells developed slowly during the extremely long larval stage, followed by rapid differentiation during a short period of metamorphosis. The excellent euryhalinity of glass eels may be due to the presence of the filament chloride cells and lamellar Na+,K+-ATPase-immunoreaction, presumably being responsible for SW and FW adaptation, respectively.





Similar content being viewed by others
References
Alderdice DF (1988) Osmotic and ionic regulation in teleost eggs and larvae. In: Hoar WS, Randall DJ (eds) Fish physiology, vol. XIA, Academic, New York, pp 163–251
Arai T, Otake T, Tsukamoto K (1997) Drastic changes in otolith microstructure and microchemistry accompanying the onset of metamorphosis in the Japanese eel Anguilla japonica. Mar Ecol Prog Ser 161:17–22
Ayson FG, Kaneko T, Hasegawa S, Hirano T (1994) Development of mitochondrion-rich cells in the yolk-sac membrane of embryos and larvae of tilapia, Oreochromis mossambicus, in fresh water and seawater. J Exp Zool 270:129–135
Ciccotti E, Macchi E, Rossi A, Cataldi E, Cataudella S (1993) Glass eel (Anguilla anguilla) acclimation to freshwater and seawater: morphological changes of the digestive tract. J Appl Ichthyol 9:74–81
Evans DH (1993) Osmotic and ionic regulation. In: Evans DH (ed) The physiology of fishes, CRC, Boca Raton, pp 315–341
Evans DH, Piermarini PM, Potts WTW (1999) Ionic transport in the fish gill epithelium. J Exp Zool 283:641–652
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid–base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177
Fricke H, Tsukamoto K (1998) Seamounts and the mystery of eel spawning. Naturwissenschaften 85:290–291
Goss CG, Laurent P, Perry SF (1992) Evidence for a morphological component in the regulation of acid–base balance in hypercapnic catfish (Ictalurus nebulosus). Cell Tissue Res 268:539–552
Guggino WB (1980) Water balance in embryos of Fundulus heteroclitus and F. bermudae adapted to seawater. Am J Physiol 238:R36–R41
Hirai N, Tagawa M, Kaneko T, Seikai T, Tanaka M (1999) Distributional changes in branchial chloride cells during freshwater adaptation in Japanese sea bass Lateolabrax japonicus. Zool Sci 16:43–49
Hiroi J, Kaneko T, Seikai T, Tanaka M (1998a) Developmental sequence of chloride cells in the body skin and gills of Japanese flounder (Paralichthys olivaceus) larvae. Zool Sci 15:455–460
Hiroi J, Kaneko T, Uchida K, Hasegawa S, Tanaka M (1998b) Immunolocalization of vascuolar-type H+-ATPase in the yolk-sac membrane of tilapia (Oreochromis mossambicus) larvae. Zool Sci 15:447–453
Hirose S, Kaneko T, Naito N, Takei Y (2003) Molecular biology of major components of chloride cells. Comp Biochem Physiol B 136:593–620
Hootman SR, Philpott CW (1979) Ultracytochemical localization of Na+, K+-activated ATPase in chloride cells from the gills of a euryhaline teleost. Anat Rec 193:99–130
Hsu SM, Raine L, Fanger H (1981) Use of avidin–biotin–peroxidase complex (ABC) in immunoperoxidase technique: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29(4):577–580
Hullet WH, Robins CR (1989) The evolutionary significance of the leptocephalus larva. In: Böhike EB (ed) Fishes of the western North Atlantic, Mem Seans Fdn Mar Res, 1(9), New Haven, pp 669–677
Hullet WH, Fischer J, Rietberg BJ (1972) Electrolyte composition of anguilliform leptocephali from the straits of Florida. Bull Mar Sci 22(2): 432–448
Hwang PP, Fang MJ, Tsai JC, Huang CJ, Chen ST (1998) Expression of mRNA and protein of Na+, K+-ATPase α-subunit in gills of tilapia (Oreochromis mossambicus). Fish Physiol Biochem 18:363–373
Kaneko T, Hasegawa S, Takagi Y, Tagawa M, Hirano T (1995) Hypoosmoregulatory ability of eyed-stage embryos of chum salmon. Mar Biol 122:165–170
Kaneko T, Shiraishi K, Katoh F, Hasegawa S, Hiroi J (2002) Chloride cells during early life stages of fish and their functional differentiation. Fish Sci 68(1):1–9
Karnaky KJ Jr, Kinter LB, Kinter WB, Stirling CE (1976) Teleost chloride cell. I. Autoradiographic localization of gill Na, K-ATPase in killifish, Fundulus heteroclitus, adapted to low and high salinity environments. J Cell Biol 70:157–177
Katoh F, Shimizu A, Uchida K, Kaneko T (2000) Shift of chloride cells distribution during early life stages in seawater-adapted Killifish, Fundulus heteroclitus. Zool Sci 17:11–18
Kawakami Y, Mochioka N, Nakazono A (1999) Immigration patterns of glass-eels Anguilla japonica entering river in northern Kyushu, Japan. Bull Mar Sci 64(2):315–327
Lasker R Threadgold LT (1968) “Chloride cells” in the skin of the larval sardine. Exp Cell Res 52:582–590
Leonard JB, Summers RG (1976) The ultrastructure of the integument of the American eel, Anguilla rostrata. Cell Tissue Res 171:1–30
Li J, Eygensteyn J, Lock RAC, Verbost PM, van der Heijden AJH, Wendelaar Bonga SE, Flik G (1995) Branchial chloride cells in larvae and juveniles of freshwater tilapia Oreochromis mossambicus. J Exp Biol 198:2177–2184
Lin H, Pfeiffer DC, Wayne vogl A, Pan J, Randall D (1994) Immunolocalization of H+-ATPase in the gill epithelia of rainbow trout. J Exp Biol 195:169–183
Lucu C, Flik G (1999) Na+, K+-ATPase and Na+/Ca2+ exchange activities in gills of hyperregulating Carcinus maenas. Am J Physiol 45:R490–R499
Marshall WS, Grosell M (2006) Ion transport, osmoregulation, and acid–base balance. In: Evans DH, Claiborne JB (eds) The physiology of fishes, 3rd edn, CRC press, Boca Raton, pp 177–230
McCormick SD (1995) Hormonal control of gill Na+, K+-ATPase and chloride cell function. In: Wood CM, Shuttleworth TJ (eds) Cellular and molecular approaches to fish ionic regulation, Academic, New York pp 285–315
Morgan IJ, Potts WTW, Oates K (1994) Intracellular ion concentrations in branchial epithelial cells of brown trout (Salmo trutta L.) determined by X-ray microanalysis. J Exp Biol 194:139–151
Nelson JS (1994) Fish of the world 3rd edn. Wiley, New York
Otake T, Ishii T, Nakahara M, Nakamura R (1994) Drastic changes in otolith strontium/calcium ratios in leptocephali and glass eels of Japanese eel Anguilla japonica. Mar Ecol Prog Ser 112:189–193
Pisam M, Massa F, Jammet C, Prunet P (2000) Chronology of the appearance of B, A, and å mitochondria-rich cells in the gill epithelium during ontogenesis of the brown trout (Salmo trutta). Anat Rec 259:301–311
Sasai S, Kaneko T, Tsukamoto K (1998a) Extrabranchial chloride cells in early life stages of the Japanese eel, Anguilla japonica. Ichthyol Res 45:95–98
Sasai S, Kaneko T, Hasegawa S, Tsukamoto K (1998b) Morphological alteration in two types of gill chloride cells in Japanese eels (Anguilla japonica) during catadromous migration. Can J Zool 76:1480–1487
Schreiber AM, Specker JL (1999) Metamorphosis in the summer flounder Paralichthys dentatus: changes in gill mitochondria-rich cells. J Exp Biol 202:2475–2484
Shelbourne JE (1957) Site of chloride regulation in marine fish larvae. Nature 180(2):920–923
Sullivan GV, Fryer JN, Perry SF (1995) Immunolocalization of proton pumps (H+-ATPase) in pavement cells of rainbow trout gill. J Exp Biol 198:2619–2629
Tabeta O, Takai T (1975a) Leptocephali of Anguilla japonica found in the waters south of the Okinawa Islands. Bull Jpn Soc Sci Fish 41(2):137–145
Tabeta O, Takai T (1975b) Leptocephalus of Anguilla japonica found in the waters south of Taiwan. Jpn J Ichthyol 22(2):100–103
Tsukamoto K (1992) Discovery of the spawning area for Japanese eel. Nature 356:789–791
Tsukamoto K (1996) Breeding place of freshwater eels. In: Tabeta O (ed) Early life history and prospects of seed production of the Japanese eel Anguilla japonica, Koseisha-Koseikaku, Tokyo pp 11–21
Tsukamoto K (2006) Spawning of eels near a seamount. Nature 439:929
Tsukamoto K, Umezawa A (1990) Early life history and oceanic migration of the eel, Anguilla japonica. La mer 28:188–198
Tsukamoto Y, Umezawa A, Tsukamoto K, Okiyama M (1992) A fully grown leptocephalus of Japanese eel collected from the western North Pacific. Nippon Suisan Gakkai shi 58(11):2209
Tsukamoto K, Nakai I, Tesch FW (1998) Do all freshwater eels migrate? Nature 396:635–636
Uchida K, Kaneko T, Yamauchi K, Hirano T (1996) Morphometrical analysis of chloride cell activity in the gill filaments and lamellae and changes in Na+, K+-ATPase activity during seawater adaptation in chum salmon fry. J Exp Zool 276:193–200
Uchida K, Kaneko T, Miyazaki H, Hasegawa S, Hirano T (2000) Excellent salinity tolerance of Mozambique tilapia (Oreochromis mossambicus): elevated chloride cell activity in the branchial and opercular epithelia of the fish adapted to concentrated seawater. Zool Sci 17(2):149–160
Ura K, Soyano K, Omoto N, Adachi S, Yamauchi K (1996) Localization of Na+, K+-ATPase in tissue of rabbit and teleosts using an antiserum directed against a partial sequence of the α-subunit. Zool Sci 13:219–227
Varsamos S, Diaz JP, Charmantier G, Blasco C, Connes R, Flik G (2002) Location and morphology of chloride cells during the post-embryonic development of the european sea bass, Dicentrarchus labrax. Anat Embryol 205:203–213
Varsamos S, Nebel C, Charmantier G (2005) Ontogeny of osmoregulation in postembryonic fish: a review. Comp Biochem Physiol A 141:401–429
Wilson JM, Laurent P, Tufts BL, Benos DJ, Donowitz M, Wayne vogl A, Randall DJ (2000) NaCl uptake by the branchial epithelium in freshwater teleost fish: an immunological approach to ion-transport protein localization. J Exp Biol 203:2279–2296
Zadunaisky J (1984) The chloride cell: the active transport of chloride and the paracellular pathways. In: Hoar WS, Randall DJ (eds) Fish physiology, vol. XB, Academic, New York pp 129–176
Acknowledgments
We are grateful to the Captain and crew of the R.V. “Tansei Maru” and R.V. “Hakuho Maru” of Ocean Research Institute, The University of Tokyo, and the R.V. “Suruga Maru” of the Shizuoka Prefectural Fisheries Experimental Station, for assistance in sampling for leptocephali and glass eels. This study was supported in part by grants-in-aid for Scientific Research (Nos. 08041139 and 11691177) from the Ministry of the Education, Culture, Sports, Science and Technology, by “Research for the Future” program No.JSPS-RFTF 97L00901 from the Japan Society for the Promotion of Science, by the Research Foundation from Touwa Shokuhin Shinkoukai, and by the Eel Research Foundation from Nobori-Kai. Partial support was also given in the form of Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists to S. S.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Nishida, Tokyo.
Rights and permissions
About this article
Cite this article
Sasai, S., Katoh, F., Kaneko, T. et al. Ontogenic change of gill chloride cells in leptocephalus and glass eel stages of the Japanese eel, Anguilla japonica . Mar Biol 150, 487–496 (2007). https://doi.org/10.1007/s00227-006-0355-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0355-8