Abstract
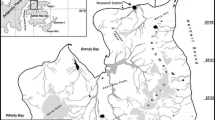
The feeding ecology of the green tiger shrimp Penaeus semisulcatus was studied in inshore fishing grounds off Doha, Qatar, using a combination of stable isotope (δ13C and δ15N) analysis and gut contents examination. Samples of post-larvae, juvenile and adult shrimp and other organisms were collected from intertidal and subtidal zones during the spawning season (January–June). Shrimp collected from shallow water seagrass beds were mostly post-larvae and juveniles and were significantly smaller than the older juveniles and adults caught in deeper macroalgal beds. Gut content examination indicated that post-larvae and juvenile shrimp in seagrass beds fed mainly on benthos such as Foraminifera, polychaetes, benthic diatoms and small benthic crustaceans (amphipods, isopods and ostracoda), whereas larger shrimp in the macroalgal beds fed mainly on bivalve molluscs and to a lesser extent polychaetes. In shrimp from both seagrass and algal beds, unidentifiable detritus was also present in the gut (18, 32%). δ13C values for shrimp muscle tissue ranged from −9.5 ± 0.26 to −12.7 ± 0.05‰, and δ15N values increased with increasing shrimp size, ranging from 4.1 ± 0.03 to 7.7 ± 0.11‰. Both δ15N values and δ13C values for shrimp tissue were consistent with the dietary sources indicated by gut contents and the δ13C and δ15N values for primary producers and prey species. The combination of gut content and stable isotope data demonstrates that seagrass beds are important habitats for post-larvae and juvenile P. semisulcatus, while the transition to deeper water habitats in older shrimp involves a change in diet and source of carbon and nitrogen that is reflected in shrimp tissue stable isotope ratios. The results of the study confirm the linkage between sensitive shallow water habitats and the key life stages of an important commercially-exploited species and indicate the need for suitable assessment of the potential indirect impacts of coastal developments involving dredging and land reclamation.






Similar content being viewed by others
References
Abed-Navandi D, Dwarschak PC (2005) Food sources of a tropical thalassinidean shrimps; a stable isotope study. Mar Ecol Prog Ser 291:150–168
Al-Ansi MA (1995) Fisheries of the state of Qatar. PhD thesis, University of Aberdeen
Al-Jamali F, Bishop JM, Osment J, Jones DA, Le Vay L (2005) A review of the impacts of aquaculture and artificial waterways upon coastal ecosystems in the Gulf (Arabian/Persian) including a case study demonstrating how future management may resolve these impacts. Aquat Ecosyst Health Manage 8:81–94
Al-Maslamani I, Le Vay L, Kennedy H (2005) Feeding in Penaeus semisulcatus at settlement; the potential role of microbial mats. In Hendy CI, Van Stappen G, Wille M, Sorgeloos P (eds) Larvi’05—4th Fish and Shellfish Larviculture Symposium, Ghent University September 5th–8th 2005. European Aquaculture Society Special Publication 36:5–8
Al-Zaidan ASY, Kennedy H, Jones DA, Al-Mohanna SY (2006) Role of microbial mats in Sulaibikhal Bay (Kuwait) mudflat food webs: evidence from δ13C analysis. Mar Ecol Prog Ser 308:27–36
Anon (1997) Review of the state of world fishery resources: marine fisheries. United Nations Food and Agriculture Organisation, Rome, Fisheries Circular No. 920 FIRM/C920
Anon (2001) Tropical shrimp fisheries and their impact on living resources. United Nations Food and Agriculture Organisation, Rome, Fisheries Circular No. 974 FIIT/C974
Basson PW, Burchard JE, Hardy JT, Price ARG (1977) Biotopes of the Western Arabian Gulf: marine life and environments of Saudi Arabia. ARAMCO, Dahran
Chansang H (1984) Mangrove detritus. In: Ong JE, Gong WK (eds) Productivity of the mangrove ecosystem: management implications. Universiti Sains Malaysia, Penang, pp 48–59
Chong VC, Sasekumar A (1981) Food and feeding habits of the white prawn P. merguiensis. Mar Ecol Prog Ser 5:185–191
Chong VC, Low CB, Ichikawa T (2001) Contribution of mangrove detritus to juvenile prawn nutrition: a dual stable isotope study in a Malaysian mangrove forest. Mar Biol 138:77–86
Cohen AC, Plisnier PD (2002) Interpreting stable isotopes in food webs: recognizing the role of time averaging at different trophic levels. Limnol Oceanogr 47(1):306–309
Coles RG, Lee Long WJ, Squire BA, Squire LC, Bibby JM (1987) Distribution of seagrass and associated juvenile commercial penaeid prawns in northeastern Queensland waters. Aust J Mar Fresh Res 38:103–120
Crocos PJ, Van der Velde TD (1995) Seasonal, spatial and interannual variability in the reproductive dynamics of the grooved tiger prawn, Penaeus semisulcatus de Haan, in Albatross Bay, Gulf of Carpentaria, Australia: the concept of effective spawning. Mar Biol 122:557–570
Dall W, Hill BJ, Rothlisberg PC, Staples DJ (eds) (1990) The biology of the Penaeidae. Adv Mar Biol 27. Academic, London
De Niro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 24:495–506
De Niro M.J, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351
Fellerhoff C, Voss M, Wantzen KM (2003) Stable carbon and nitrogen isotope signatures of decomposing tropical macrophytes. Aquatic Ecol 37(4):361–375
Fontugne MR, Duplessy JC (1981) Organic carbon isotope fractionation by marine plankton in the temperature range −1 to 31°C. Oceanolog Acta 4:85–90
Fry B (1981) Natural stable carbon isotope tag traces Texas shrimp migration. Fish Bull US 79:11–19
Fry B, Parker PL (1979) Animals diet in Texas seagrass meadows δ13C evidence for the importance of benthic plants. Estuar Coast Mar Sci 8:499–509
Fry B, Quiñones RB (1994) Biomass spectra and stable isotope indicators of trophic level in zooplankton of the northwest Atlantic. Mar Ecol Prog Ser 112:201–204
Fry B, Mumford PL, Robblee M B (1999) Stable isotope studies of pink shrimp (Farfantepenaeus durorarum Burkenroad) migrations on the southwestern Florida Shelf. Bull Mar Sci 65:419–430
Haywood MDE, Vance DJ, Loneragan NR (1995) Seagrass and algal beds as nursery habitats for tiger prawns (Penaeus semisulcatus and P. esculentus) in a tropical Australian estuary. Mar Biol 122:213–223
Heales DS (2000) The feeding of juvenile grooved tiger prawns Penaeus semisulcatus in a tropical Australian Estuary: a comparison of diets in intertidal seagrass and subtidal algal beds. Asian Fish Sci 13(2) (abstract)
Heales DS, Vance DJ, Loneragan NR (1996) Field observation of moult cycle, feeding behaviour, and diet of small juvenile tiger prawns Penaeus semisulcatus in a tropical seagrass bed in the Embley River. Aust Mar Fresh Res 145:43–51
Hemminga MA, Mateo MA (1996) Stable carbon isotopes in seagrasses: variability in ratios and use in ecological studies. Mar Ecol Prog Ser 140:285–298
Hill BJ (1976) Natural food, foregut clearance rate and activity of the crab Scylla serrata. Mar Biol 34:109–116
Hill BJ, Wassenberg TJ (1993) Why are some prawn found in seagrass? An experimental study of brown (Penaeus esculentus) and grooved (Penaeus semisulcatus) tiger prawns. Aust Mar Fresh Res 44:221–227
Hunter J (1984) Immunological dietary analysis and diel feeding chronology for Penaeus aztecus (Ives) and Penaeus setiferus (L.) in tide creeks of North Inlet, South Carolina. M.S. Thesis, University of South Carolina, Columbia South Carolina creek. J Exp Mar Biol Ecol:61–70
Jackson CJ, Rothlisberg PC, Pendrey RC (2001) Role of larval distribution and abundance in the overall life-history dynamics: a study of the prawn Penaeus semisulcatus in Albatross Bay, Gulf of Carpentaria, Australia. Mar Ecol Prog Ser 213:241–252
Jones DA, Al-Attar M (1981) Observations on the postlarval and juvenile habitats of Penaeus semisulcatus in Kuwait Bay and adjacent waters. In: Mathews CP (ed) Revised proceedings, shrimp fisheries management workshop. Kuwait Institute for Scientific Research Report No. 670:112–129
Jones DA, Price ARG, Al-Yamani F, Al-Zaidan A (2002) Costal and marine ecology. In: Kahn NY, Munwar M, Price ARG (eds) The Gulf ecosystem; health and sustainability. Ecovision World Monograph Series, Bakhuys, Leiden, pp 65–103
Khan NY, Munwar M, Price ARG (2002) Environmental trends and integrated management of the Gulf. In: Khan NY, Munwar M, Price ARG (eds) The Gulf ecosystem; health and sustainability. Backhuys, Leiden, pp 483–494
Leber KM (1985) Influence of decapod foraging and microhabitat complexity on seagrass communities: a field test of the refuge hypothesis. Ecology 66:1951–1964
Loneragan NR, Bunn SE, Kellaway DM (1997) Are mangroves and seagrasses sources of organic carbon for penaeid prawns in a tropical Australian estuary ? A multiple stable isotope study. Mar Biol 130:289–300
Loneragan NR, Kenyon RA, Haywood MDE, Staples DJ (1994) Population dynamic of juvenile tiger prawns (Penaeus esculentus and Penaeus semisulcatus) in seagrass habitats of the western Gulf of Carpentaria, Australia. Mar Biol 119:133–143
Loneragan NR, Kenyon RA Staples DJ Poiner IR, Conacher CA (1998) The influence of seagrass type on the distribution and abundance of postlarval and juvenile tiger prawns (Penaeus esculentus and P. semisulcatus) in the western Gulf of Carpentaria, Australia. J Exp Mar Biol Ecol 228:175–195
Marguillier S, Velde G, Dehairs F, Hemminga MA, Rajagopal S (1997) Trophic relationships in an interlinked mangrove seagrass ecosystem as traced by δ13C and δ15N. Mar Ecol Prog Ser 151:115–121
Mayer MA (1985) Ecology of juvenile white shrimp, Penaeus setiferus Linnaeus, in the salt marsh habitat. Masters Thesis, Georgia Institute of Technology, Atlanta
McCutchan JH, Lewis WM, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen and sulfur. Oikos 102:378–390
McTigue TA, Zimmerman RJ (1991) Carnivory versus herbivory in juvenile Penaeus setiferus (Linnaeus) and Penaeus aztecus (Ives). J Exp Mar Biol Ecol 151:1–16
Minagawa M, Wada E (1984) Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim Cosmochim Acta 48:1135–1140
Mohammed MA, Bishop JM, Yimin Y (1998) Kuwait’s post Gulf-war shrimp fishery and stock status from 1991/92 Through 1995/96. Rev Fish Sci 6:253–280
Mohan PC, Rao RG, Dehairs F (1997) Role of Godavari mangroves (India) in the production and survival of prawn larvae. Hydrobiologia 358:317–320
Moncreiff CA, Sullivan MJ (2001) Trophic importance of epiphytic in subtropical seagrass beds: evidence from multiple stable isotope analyses. Mar Ecol Prog Ser 215:93–106
Nelson WG, Capone MA (1990) Experimental studies of predation on polychaetes associated with seagrass beds. Estuaries 13:51–58
O’Brien CJ. (1994) Ontogenetic change in the diet of juvenile brown tiger Penaeus esculentus. Mar Ecol Prog Ser 112:195–200
Parker PL, Calder JA (1970) Stable carbon isotope ratio variations in biological systems. In: Hood DW (eds) Organic matter in natural waters. University of Alaska Press, Occas Publ 1:107–127
Parker PL, Anderson RK, Lawrence A (1989) A δ 13C and δ 15N tracer study of nutrition in aquaculture: Penaeus vannamei in a pond growout system. In: Rundel PW, Ehleringer JR, Nagy KA (eds) Stable isotopes in ecological research. Springer, Berlin Heidelberg New York, pp 288–303
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Ann Rev Ecol Syst 18:293–320
Primavera JH (1996) Stable carbon and nitrogen isotope ratios of penaeid juveniles and primary producers in a riverine mangrove in Guimaras, Philippines. Bull Mar Sci 58:675–683
Rönnbäck P, Macia A, Almqvist G, Schutz L, Troell M (2002) Do penaeid shrimp have a preference for mangrove habitats? Distribution pattern analysis on Inhaca Island, Mozambique. Estuar Coast Shelf Sci 55:427–436
Rothlisberg PC (1998) Aspects of penaeid biology and ecology of relevance to aquaculture: a review. Aquaculture 164:49–65
Schwamborn R, Criales MM (2000) Feeding strategy and daily ratio of juvenile pink shrimp (Farfantepenaeus duorarum) in a South Florida seagrass bed. Mar Biol 137:139–147
Sheppard C, Price A, Roberts C (1992) Marine ecology of the Arabian Region. Pattern and processes in extreme tropical environment. Academic, London
Somers IF (1994) Species composition and distribution of commercial penaeid prawn catches in the Gulf of Carpentaria, Australia, in relation to depth and sediment type. Aust J Mar Fresh Res 45:317–335
Somers I.F, Kirkwood G (1991) Population ecology of the grooved tiger prawn, Penaeus semisulcatus, in the north-western Gulf of Carpentaria, Australia: growth, movement, age structure and infestation by the bopyrid parasite Epipeneon ingens. Aust J Mar Fresh Res 42:349–367
Staples DJ, Vance DJ, Heales DS (1985) Habitat requirements of juvenile penaeid prawns and their offshore fisheries. In: Rothlisberg PC, Hill BJ, Staples DJ (eds) Second Australian nation prawn seminar NPS2. CSIRO Cleveland Marine Laboratories, Cleveland, pp 47–54
Stoner AW, Zimmerman RJ (1988) Food pathways associated with penaeid shrimps in a mangrove-fringed estuary. Fish Bull US 86:543–555
Thayer GW, Parker PL, La Croix MW, Fry B (1978) The stable carbon isotope ratio of some components of an eelgrass, Zostera marina, bed. Oecologia 35:1–12
Van Dover CL (2002) Tropic relationships among invertebrates at the Kairei hydrothermal vent field (Central Indian Ridge). Mar Biol 141:761–772
Vanderklift MA, Ponsard S (2003) Sources of variation in consumer-diet 15N enrichment: a meta-analysis. Oecologia 136:169–182
Wassenberg TJ, Hill BJ (1987) Natural diet of tiger prawns Penaeus esculentus and P. semisulcatus. Aust J Mar Fresh Res 38:169–182
Yokoyama H, Tamaki A, Harada K, Shimoda K, Koyama K, Ishihi Y (2005) Variability of diet-tissue isotopic fractionation in estuarine macrobenthos. Mar Ecol Prog Ser 296:115–128
Young PC, Carpenter SM (1977) Recruitment of postlarval prawns to nursery areas in Moreton Bay, Queensland. Aust J Mar Fresh Res 28:745–773
Zimmerman RJ, Minello TJ, Zamora G (1984) Selection of vegetated habitat by brown shrimp, Penaeus aztecus, in a Galveston Bay salt marsh. Fish Bull US 84:325–336
Acknowledgments
The authors express their thanks to Dr Ian Lucas for help with identification of microbial mat flora and Paul Kennedy for help with stable isotope and C & N analyses. We are grateful to Abdul Latif Al-Maslamani, Dr Jassem Al-Khayat and boatman Mutiez Ahmed for their help in the field work in Qatar. This research was supported by a PhD scholarship from the State of Qatar to Ibrahim Al-Maslamani.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Thorpe, Port Erin
Rights and permissions
About this article
Cite this article
Al-Maslamani, I., Le Vay, L., Kennedy, H. et al. Feeding ecology of the grooved tiger shrimp Penaeus semisulcatus De Haan (Decapoda: Penaeidae) in inshore waters of Qatar, Arabian Gulf. Mar Biol 150, 627–637 (2007). https://doi.org/10.1007/s00227-006-0346-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0346-9