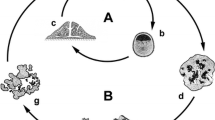
Abstract
We performed a screening on 21 Mediterranean sponge species in order to know from which ones primmorphs can be obtained, with a comparison of their formation among the outputs. Thirteen species produced primmorphs, evidencing a high variability among species concerning the number and size of primmorphs, without evidencing any taxonomical specificity. Additionally, the formation process and trend of growth during 1 month of monitoring were studied from a quantitative point of view. The dynamics of primmorphs formation follow different patterns: slow and quick formation, short and long-time survival and in the case of long-time survival, the fission and fusion models are proposed. Besides these patterns, each analysed species showed characteristic trends in the time required for the aggregation of cells, the time required for the production of small primmorphs, growth fluctuation and the acquirement of a final stable size.







Similar content being viewed by others
References
Borojevic R, Fry WG, Jones WC, Levi C, Rasmont R, Sarà M, Vacelet J (1967) Mise au point actuelle de la terminologie des éponges (A reassessment of the terminology for sponges). Bullettin du Museum National D’Histoire Naturelle 2e Série, Tome 39, No. 6, pp 1224–1235
Custodio MR, Prokic I, Steffen R, Koziol C, Borojevic R, Brümmer F, Nickel M, Müller WEG (1998) Primmorphs generated from dissociated cells from the sponge Suberites domuncula: a model system for studies of cell proliferation and cell death. Mech Ageing Dev 105:45–59
De Caralt S, Agell G, Uriz MJ (2003) Long-term culture of sponge explants: conditions enhancing survival and growth and assessment of bioactivity. Biomol Eng 20:339–347
De Rosa S, De Caro S, Iodice C, Tommonaro G, Stefanov K, Popov S (2003) Development in primary cell culture of demosponges. J Biotechnol 100:119–125
Diaz J (1979) Variations, differenciations et fonctions des catégories cellulaires de la demosponge d’eau saumaitre Suberites massa Nardo, au cours du cycle biologique annuel et dans des conditons expérimentales. Thèse, Univ Sci Tech Languedoc, pp 1–332
Gaino E, Bavestrello G, Magnino G (1999) Self/non-self recognition in sponges. Ital J Zool 66:299–315
Ilan M, Contini H, Carmeli S, Rinkevich B (1996) Progress towards cell cultures from a marine sponge that produces bioactive compounds. J Mar Biotechnol 4:145–149
Klatau M, Custodio MR, Borojevic R (1994) In vitro culture of primary cell lines from marine sponges. In: van Soest R, van Kempen TMG, Brakman JC (eds) Sponges in time and space. AA Balkema, Rotterdam, pp 401–406
Koziol C, Borojevic R, Steffen R, Müller WEG (1998) Sponges (Porifera) model systems to study the shift from immortal to senescent somatic cells: the telomerase activity in somatic cells. Mech Ageing Dev 100:107–120
Krasko A, Batel R, Schroeder HC, Müller IM, Müller WEG (2000) Expression of silicatein collagen genes in the marine sponge Suberites domuncula is controlled by silicate and myotrophin. Eur J Biochem 167:4878–4887
Müller WEG, Steffen R, Rinkevich B, Matranga V, Kurelec B (1996) The multixenobiotic resistance mechanism in the marine sponge Suberites domuncula: its potential applicability for the evaluation of environmental pollution by toxic compounds. Mar Biol 125:165–170
Müller WEG, Wiens M, Batel R, Steffen R, Schroeder HC, Borojevic R, Custodio MR (1999) Establishment of a primary cell culture from a sponge: primmorphs from Suberites domuncula. Mar Ecol Prog Ser 178:205–219
Müller WEG, Bohm M, Batel R, De Rosa S, Tommonaro G, Müller IM, Schroeder HC (2000) Application of cell culture for the production of bioactive compounds from sponges: synthesis of avarol from Dysidea avara. J Nat Prod 63:1077–1081
Müller WEG, Wiens M, Adell T, Gamulin V, Schroeder HC, Müller IM (2004a) Bauplan of Urmetazoa: basis for genetic complexity of Metazoa. Int Rev Cytol 235:53–92
Müller WEG, Wiens M, Müller IM, Brümmer F (2004b) From cell to primmorphs and adult sponges: an approach to understand the bauplan of Demosponges. In: Pansini M, Pronzato R, Bavestrello G, Manconi R (eds) Sponge science in the new millennium. Boll Mus Ist Biol Univ Genova 68:39–54
Nickel M, Brümmer F (2003) In vitro sponge fragment culture of Chondrosia reniformis (Nardo, 1847). J Biotechnol 100:147–159
Osinga R, Tramper J, Wijffels RH (1999) Cultivation of marine sponges. Mar Biotechnol 1:509–532
Pomponi SA, Willoughby R (1994) Sponge cell culture for production of bioactive metabolites. In: Van Soest R, Van Kempen TMG, Brakman JC (eds) Sponge in time and space. Balkema, Rotterdam, pp 395–400
Pozzolini M, Sturla L, Cerrano C, Bavestrello G, Camardella L, Parodi AM, Raheli F, Benfatti U, Müller WEG, Giovine M (2004) Molecular cloning of silicate in gene from the Sponge Petrosia ficiformis (Porifera, demospongia) and development of primmorphs as a model for biosilification. Mar Biotechnol 6:594–603
Pronzato R, Cerrano C, Cubeddu G, Magnino G, Manconi R, Pastore S, Sarà A, Sidri M (1999) The millennial history of commercial sponges: from harvesting to farming and integrate aquaculture. Biol Mar Mediterr 7(2):1–12
Richelle-Maurer E, Gomez R, Braekman JC, Van de Vyver G, Van Soest RWM, Devijver C (2003) Primary cultures from the marine sponge Xestospongia muta (Petrosiidae, Haplosclerida). J Biotechnol 100:169–176
Rinkevich B (1999) Cell culture from marine invertebrates: obstacles, new approaches and recent improvements. J Biotechnol 70:133–153
Sarà M, Vacelet J (1973) Ecologie des Demosponges. In: Grasseé (ed) Traité de Zoologie, Anatomie, Sistematique, Biologie, III, 1, pp 462–546
Siegel S, Castellan NJ (1988) Statistica non parametrica. Caracciolo Editore
Sipkema D, Van Wielink R, Van Lammeren AAM, Tramper J, Osinga R, Wijffels RH (2003) Primmorphs from seven marine sponges: formation and structure. J Biotechnol 100:127–139
Turon X, Tarjuelo I, Uriz M (1998) Growth dynamics and mortality of the encrusting sponge Crambe crambe (Poecilosclerida) in contrasting habitats: correlation with population structure and investment in defence. Funct Ecol 12:631–639
Van Treeck P, Eisinger M, Müller J, Paster M, Schumacher H (2003) Mariculture trials with Mediterranean sponge species. Aquaculture 218:349–455
Von Lange T (1998) Telomeres and senescence: ending the debate. Science 279:334–335
Wegner C, Steffen R, Koziol C, Batel R, Lacorn M, Steinbart H, Simat T, Müller WEG (1998) Apoptosis in marine sponges: a biomarker for environmental stress (cadmium and bacteria). Mar Biol 131:411–421
Wilson HV (1907) On some phenomena of coalescence and regeneration in sponges. J Exp Zool 5:245–258
Zhang W, Zhang X, Cao X, Xu J, Zhao Q, Yu X, Jin M, Deng M (2003) Optimizing the formation of in vitro sponge primmorphs from the Chinese sponge Stylotella agminata (Ridley). J Biotechnol 100:161–168
Acknowledgements
We would like to thank M. Bertolino (University of Genoa, Italy) for field assistance and sponges identification and M. Sidri for her helpful suggestions. This work was supported by Italian MIUR funds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Cattaneo-Vietti, Genova
Rights and permissions
About this article
Cite this article
Valisano, L., Bavestrello, G., Giovine, M. et al. Primmorphs formation dynamics: a screening among Mediterranean sponges. Mar Biol 149, 1037–1046 (2006). https://doi.org/10.1007/s00227-006-0297-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0297-1