Abstract
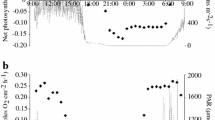
We investigated heterogeneity of light acclimation of photosynthesis in sun- and shade-adapted coenosarc and polyp tissues of Pocillopora damicornis. The zooxanthellar community within P. damicornis colonies at Heron Island is genetically uniform, yet they showed a large degree of plasticity in their photo-physiological acclimation linked to light microclimates characterised by fibre-optic microprobes. Microscale scalar irradiance measurements showed higher absorption in polyp than coenosarc tissues and higher absorption in the more densely pigmented shade-adapted polyps than in sun-adapted polyps. The combination of an O2 microelectrode with a fibre-optic microprobe (combined sensor diameter 50–100 μm) enabled parallel measurements of O2 concentration, gross photosynthesis rate and photosystem II (PSII) quantum yield at the coral surface under steady-state conditions as a function of increasing irradiances. Lower O2 levels at the tissue surface and higher compensation irradiance indicated a higher respiration activity in sun-adapted polyp tissue as compared to shade-adapted polyps. Shade-adapted coenosarc and polyp tissues exhibited lower maxima of relative electron transport rates (rETRmax) (84±15 and 41±10, respectively) than sun-adapted coenosarc and polyp tissues (136±14 and 77±13, respectively). Shade-adapted tissues showed stronger decrease of rETR at high scalar irradiances as compared to sun-adapted tissues. The relationship between the relative PSII electron transport and the rate of gross photosynthesis, as well as O2 concentration, was non-linear in sun-adapted tissues over the entire irradiance range, whereas for shade-adapted tissues the relationship became non-linear at medium to high scalar irradiances >200 μmol photons m−2 s−1. This suggests that rETR measurements should be used with caution in corals as a proxy for photosynthesis rates. The apparently high rates of photosynthesis (oxygen evolution rates) suggest that there must be a considerable electron transport rate through the photosystems that is not observed by the rETR measurements. This may be accounted for by vertical heterogeneity of zooxanthellae in the tissue and the operation of an alternative electron pathway such as cyclic electron flow around PSII.








Similar content being viewed by others
References
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Baker AC, Starger CJ, McClanahan TR, Glynn PW (2004) Corals’ adaptive response to climate. Nature 430:741
Bhagooli R, Hidaka M (2003) Comparison of stress susceptibility of in hospite and isolated zooxanthellae among five coral species. J Exp Mar Biol Ecol 291:181–197
Brown BE, Downs CA, Dunne RP, Gibb SW (2002) Preliminary evidence for tissue retraction as a factor in photoprotection of corals incapable of xanthophyll cycling. J Exp Mar Biol Ecol 277:129–144
Buddemeier RW, Fautin DG (1993) Coral bleaching as an adaptive mechanism. BioScience 48:320–326
Coles SL, Jokiel PL (1977) Effects of temperature on photosynthesis and respiration in hermatypic corals. Mar Biol 43:209–216
De Beer D, Kühl M, Stambler N, Vaki L (2000) A microsensor study of light enhanced Ca2+ uptake and photosynthesis in the reef-building coral Favia sp. Mar Ecol Prog Ser 194:75–85
Dubinsky Z, Falkowski PG, Porter JW, Muscatine L (1984) Absorption and utilization of radiant energy by light- and shade adapted colonies of the hermatypic coral Stylophora pistillata. Proc R Soc Lond B 222:203–214
Enriquez S, Méndez ER, Iglesias-Prieto R (2005) Multiple scattering on coral skeletons enhances light absorption by symbiotic algae. Limnol Oceanogr 50(4):1025–1032
Falkowski PG, Dubinsky Z, Muscatine L, Porter JW (1984) Light and the bioenergetics of a symbiotic coral. BioScience 34:705–709
Falkowski PG, Jokiel PL, Kinzie RA (1990) Irradiance and corals. In: Dubinsky Z (ed) Ecosystems of the World 25: Coral Reefs. Elsevier, Amsterdam, pp 89–107
Forster RM, Kromkamp JC (2004) Modelling the effects of chlorophyll fluorescence from subsurface layers on photosynthetic efficiency measurements in microphytobenthic algae. Mar Ecol Prog Ser 284:9–22
Franklin LA, Badger MR (2001) A comparison of photosynthetic electron transport rates in macroalgae measured by pulse amplitude modulated chlorophyll fluorometry and mass spectrometry. J Phycol 37:756–767
Gattuso J-P, Jaubert J (1990) Effect of light on oxygen and carbon dioxide fluxes and on metabolic quotients measured in situ in a zooxanthellate coral. Limnol Oceanogr 35(8):1796–1804
Geel C, Versluis W, Snel JFH (1997) Estimation of oxygen evolution by marine phytoplankton from measurements of the efficiency of photosystem II electron flow. Photosynth Res 51:61–70
Harbinson J, Genty B, Baker NR (1990) The relationship between CO2 assimilation and electron transport in leaves. Photosynth Res 25:213–224
Hill R, Schreiber U, Gademann R, Larkum AWD, Kühl M, Ralph PJ (2004) Spatial heterogeneity of photosynthesis and the effect of temperature-induced bleaching conditions in three species of coral. Mar Biol 144:633–640
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50:839–869
Jones RJ, Hoegh-Guldberg O, Larkum AWD, Schreiber U (1998) Temperature-induced bleaching of corals begins with impairment of the CO2 mechanism in zooxanthellae. Plant Cell Environ 21:1219–1230
Kühl M (2005) Optical microsensors for analysis of microbial communities. Methods Enzymol 397:166–199
Kühl M, Jørgensen BB (1994) The light field of micro-benthic communities: radiance distribution and microscale optics of sandy coastal sediments. Limnol Oceanogr 39:1368–1398
Kühl M, Cohen Y, Dalsgaard T, Jørgensen BB, Revsbech NP (1995) Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studies with microsensors for O2, pH and light. Mar Ecol Prog Ser 117:159–172
Kühl M, Glud RN, Ploug H, Ramsing NB (1996) Microenvironmental control of photosynthesis and photosynthesis-coupled respiration in an epilithic cyanobacterial biofilm. J Phycol 32:799–812
Kühl M, Glud RN, Borum J, Roberts R, Rysgaard S (2001) Photosynthetic performance of surface associated algae below sea ice as measured with a pulse amplitude modulated (PAM) fluorometer and O2 microsensors. Mar Ecol Prog Ser 223:1–14
LaJeunesse TC, Loh WKW, van Woesik R (2003) Low symbiont diversity in southern Great Barrier Reef corals, relative to those of the Caribbean. Limnol Oceanogr 48(5):2046–2054
Lassen C, Plough H, Jørgensen BB (1992) A fibre-optic scalar irradiance microsensor: application for spectral light measurements in sediments. FEMS Microbiol Ecol 86:247–254
Lavaud J, van Gorkom H, Etienne A (2002) Photosystem II electron transfer cycle and chlororespiration in planktonic diatoms. Photosynth Res 74:51–59
Lesser MP, Mazel C, Phinney D, Yentsch CS (2000) Light absorption and utilization by colonies of the congeneric hermatypic corals Montastraea faveolata and Montastraea cavernosa. Limnol Oceanogr 45(1):76–86
Longstaff BJ, Kildea T, Runcie JW, Cheshire A, Dennison WC, Hurd C, Kana T, Raven JA, Larkum AWD (2002) An in situ study of photosynthetic oxygen exchange and electron transport rate in the marine macroalga Ulva lactuca (Chlorophyta). Photosynth Res 74:281–293
Lorenzen J, Glud R, Revsbech NP (1995) Impact of microsensor-caused changes in diffusive boundary layer thickness on O2 profiles and photosynthetic rates in benthic communities of microorganisms. Mar Ecol Prog Ser 199:237–241
Marshall PA, Baird AH (2000) Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19:155–163
Patterson MR (1992) A chemical engineering view of cnidarian symbioses. Am Zool 32(4):566–582
Patterson MR, Sebens KP, Olson RR (1991) In situ measurements of flow effects on primary production and dark respiration in reef corals. Limnol Oceanogr 36:936–948
Perkins RG, Oxborough K, Hanlon ARM, Underwood GJC, Baker NR (2002) Can chlorophyll fluorescence be used to estimate the rate of photosynthetic electron transport within microphytobenthic biofilms. Mar Ecol Prog Ser 228:47–56
Platt T, Gallegos CL, Harrison WG (1980) Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
Ralph PJ, Gademann R (2005) Rapid light curves: a powerful tool for the assessment of photosynthetic activity. Aquat Bot 82:222–237
Ralph PJ, Gademann R, Larkum AWD, Kühl M (2002) Spatial heterogeneity in active chlorophyll fluorescence and PSII activity of coral tissues. Mar Biol 141:639–646
Ralph PJ, Schreiber U, Gademann R, Kühl M, Larkum AWD (2005) Coral photobiology studied with a new imaging pulse amplitude modulated fluorometer. J Phycol 41:335–342
Revsbech NP (1989) An oxygen microelectrode with a guard cathode. Limnol Oceanogr 34:474–478
Revsbech NP, Jørgensen BB (1983) Photosynthesis of benthic microflora measured with high spatial resolution by the oxygen microprofile method: capabilities and limitations of the method. Limnol Oceanogr 28:749–756
Salih A, Larkum AWD, Cox G, Kühl M, Hoegh-Guldberg O (2000) Fluorescent pigments in corals are photoprotective. Nature 408:850–853
Schnitzler-Parker M, Armbrust EV, Piovia-Scott J, Keil RG (2004) Induction of photorespiration by light in the centric diatom Thalassiosira weissflogii (Bacillariopyceae): molecular characterization and physiological consequences. J Phycol 40:557–567
Schreiber U (2004) Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview. In: Papageorgiou GC, Govindjee (eds) Chlorophyll fluorescence: a signature of photosynthesis. Kluwer Academic Publishers, Dordrecht, pp. 279–319
Schreiber U, Kühl M, Klimant I, Reising H (1996) Measurement of chlorophyll fluorescence within leaves using a modified PAM fluorometer with a fiber-optic microprobe. Photosynth Res 47:103–109
Shashar N, Cohen Y, Loya Y (1993) Extreme fluctuations of oxygen in diffusive boundary layers surrounding stony corals. Biol Bull 185:455–461
Stambler N, Dubinsky Z (2004) Corals as light collectors: an integrating sphere approach. Coral Reefs 24(1):1–9
Ulstrup KE, Hill R, Ralph PJ (2005) Photosynthetic impact of hypoxia on in hospite zooxanthellae in the scleractinian coral Pocillopora damicornis. Mar Ecol Prog Ser 286:125–132
Ulstrup KE, Berkelmans R, Ralph PJ, van Oppen MJH (2006) Variation in bleaching sensitivity of two coral species with contrasting bleaching thresholds across a latitudinal gradient on the GBR. Mar Ecol Prog Ser (in press)
Warner ME, Fitt WK, Schmidt GW (1999) Damage to photosystem II in symbiotic dinoflagellates: a determinant bleaching. Proc Natl Acad Sci USA 96:8007–8012
Acknowledgements
We thank A. Glud for microsensor construction, N. Ralph for fabricating the flow-through chamber, and Dr B. Kelaher for advice on statistical data processing. We thank S.H. Magnusson for assistance with collecting the scalar irradiance data. We acknowledge R. Hill for editorial comments on drafts of the manuscript and the Department of Environmental Science, UTS and all staff at Heron Island Research Station for support. The research was funded jointly by a GBRMPA Science for Management award and a PADI grant to KEU, a UTS institutional grant to PJR, grants from the Australian Research Council to AWDL and PJR, and a grant from the Danish Natural Science Research Council to MK. The research was conducted under GBRMPA permit number G04/12776.1. This is contribution number 207 from the Institute of Water and Resource Management.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G.F. Humphrey, Sydney
Rights and permissions
About this article
Cite this article
Ulstrup, K.E., Ralph, P.J., Larkum, A.W.D. et al. Intra-colonial variability in light acclimation of zooxanthellae in coral tissues of Pocillopora damicornis . Mar Biol 149, 1325–1335 (2006). https://doi.org/10.1007/s00227-006-0286-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0286-4