Abstract
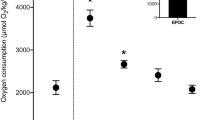
The European seabass is an active euryhaline teleost that migrates and forages in waters of widely differing salinities. Oxygen uptake (MO2) was measured in seabass (average mass and forklength 510 g and 34 cm, respectively) during exercise at incremental swimming speeds in a tunnel respirometer in seawater (SW) at a salinity of 30‰ and temperature of 14°C, and their maximal sustainable (critical) swimming speed (Ucrit) determined. Cardiac output (Q) was measured via an ultrasound flow probe on their ventral aorta. The fish were then exposed to acute reductions in water salinity, to either SW (control), 10‰, 5‰, or freshwater (FW, 0‰), and their exercise and cardiac performance measured again, 18 h later. Seabass were also acclimated to FW for 3 weeks, and then their exercise performance measured before and at 18 h after acute exposure to SW at 30‰. In SW, seabass exhibited an exponential increase in MO2 and Q with increasing swimming speed, to a maximum MO2 of 339±17 mg kg−1 h−1 and maximum Q of 52.0±1.9 ml min−1 kg−1 (mean±1 SEM; n=19). Both MO2 and Q exhibited signs of a plateau as the fish approached a Ucrit of 2.25±0.08 bodylengths s−1. Increases in Q during exercise were almost exclusively due to increased heart rate rather than ventricular stroke volume. There were no significant effects of the changes in salinity upon MO2 during exercise, Ucrit or cardiac performance. This was linked to an exceptional capacity to maintain plasma osmolality and tissue water content unchanged following all salinity challenges. This extraordinary adaptation would allow the seabass to maintain skeletal and cardiac muscle function while migrating through waters of widely differing salinities.




Similar content being viewed by others
References
Axelsson M, Davison W, Forster ME, Farrell AP (1992) Cardiovascular-responses of the red-blooded Antarctic fishes Pagothenia bernacchii and P borchgrevinki. J Exp Biol 167:179–201
Axelsson M, Altimiras J, Claireaux G (2002) Post-prandial blood flow to the gastrointestinal tract is not compromised during hypoxia in the seabass Dicentrarchus labrax. J Exp Biol 205:2891–2896
Bath RN, Eddy FB (1979) Salt and water balance in rainbow trout Salmo gairdneri rapidly transferred from freshwater to seawater. J Exp Biol 83:193–202
Beaumont MW, Butler PJ, Taylor EW (2003) Exposure of brown trout, Salmo trutta, to a sub-lethal concentration of copper in soft acidic water: effects upon gas exchange and ammonia accumulation. J Exp Biol 206:153–162
Brauner CJ, Shrimpton JM, Randall DJ (1992) The effect of short-duration seawater exposure on plasma ion concentrations and swimming performance in coho salmon (Oncorhynchus kisutch). Can J Fish Aquat Sci 49:2399–2405
Brauner CJ, Iwama GK, Randall DJ (1994) The effect of short-duration seawater exposure on the swimming performance of wild and hatchery-reared juvenile coho salmon (Oncorhynchus kisutch) during smoltification. Can J Fish Aquat Sci 51:2188–2194
Brett JR (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Can 21:1183–1226
Brill RW, Bushnell PG (2001) The cardiovascular system of tunas. In: Block BA, Stevens ED (eds) Tuna: physiology, ecology and evolution. Academic, San Diego, pp 79–120
Claireaux G, Audet C (2000) Seasonal changes in hyperosmoregulatory ability of brook char: the role of environmental factors. J Fish Biol 56:347–373
Claireaux G, Lagardère JP (1999) Influence of temperature, oxygen and salinity on the metabolism of the European seabass. J Sea Res 42:157–168
Claireaux G, McKenzie DJ, Genge G, Chatelier A, Aubin J, Farrell AP (2005) Linking swimming performance, cardiac performance and cardiac morphology in rainbow trout. J Exp Biol (in press)
Day N, Butler PJ (1996) Environmental acidity and white muscle recruitment during swimming in the brown trout (Salmo trutta). J Exp Biol 199:1947–1959
Farrell AP (1996) Features heightening cardiovascular performance in fishes, with special reference to tunas. Comp Biochem Physiol 113A:61–67
Farrell AP (2002) Cardiorespiratory performance in salmonids during exercise at high temperature: insights into cardiovascular design limitations in fishes. Comp Biochem Physiol 132A:797–810
Farrell AP, Jones DR (1992) The heart. In: Hoar WS, Randall DJ, Farrell AP (eds) Fish physiology, vol 12A. Academic, New York, pp 1–88
Fry FEJ (1971) The effect of environmental factors on the physiology of fish. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 6. Academic, New York, pp 1–99
Gallaugher PE, Thorarensen H, Kiessling A, Farrell AP (2001) Effects of high intensity exercise training on cardiovascular function, oxygen uptake, internal oxygen transfer and osmotic balance in chinook salmon (Oncorhynchus tshawytscha) during critical speed swimming. J Exp Biol 204:2861–2872
Hwang PP, Sun CM, Wu SM (1989) Changes in plasma osmolality, chloride concentration and gill Na-K-ATPase activity in tilapia Oreochromis mossambicus during seawater adaptation. Mar Biol 100:295–300
Jensen FB, Lecklin T, Busk M, Bury NR, Wilson RW, Wood CM, Grosell M (2002) Physiological impact of salinity increase at organism and red blood cell levels in the European flounder (Platichthys flesus). J Exp Mar Biol Ecol 274:159–174
Jensen MK, Madsen SS, Kristiansen K (1998) Osmoregulation and salinity effects on the expression and activity of Na+,K+-ATPase in the gills of European seabass, Dicentrarchus labrax (L.). J Exp Zool 282:290–300
Kiceniuk JW, Jones DR (1977) The oxygen transport system in trout (Salmo gairdneri) during sustained exercise. J Exp Biol 69:247–260
Kolok AS, Farrell AP (1994a) Individual variation in the swimming performance and cardiac performance of northern squawfish, Ptychocheilus oregonensis. Physiol Zool 67:706–722
Kolok AS, Farrell AP (1994b) The relationship between maximum cardiac output and swimming performance in northern squawfish, Ptychocheilus oregonensis: the effect of coronary artery ligation. Can J Zool 72:1687–1690
Kolok AS, Sharkey D (1997) Effect of freshwater acclimation on the swimming performance and plasma osmolarity of the euryhaline gulf killifish. Trans Am Fish Soc 126:866–870
Kolok AS, Spooner RM, Farrell AP (1993) The effect of exercise on the cardiac output and blood flow distribution of the largescale sucker Catostomus macrocheilus. J. Exp Biol 183:301–321
Korsmeyer KE, Lai NC, Shadwick RE, Graham JB (1997a) Heart rate and stroke volume contributions to cardiac output in swimming yellowfin tuna: response to exercise and temperature. J Exp Biol 200:1975–1986
Korsmeyer KE, Lai NC, Shadwick RE, Graham JB (1997b) Oxygen transport and cardiovascular responses to exercise in yellowfin tuna Thunnus albacores. J Exp Biol 200:1987–1997
McKenzie DJ, Cataldi E, Owen S, Taylor EW, Bronzi P (2001a) Effects of acclimation to brackish water on the growth, respiratory metabolism and exercise performance of Adriatic sturgeon (Acipenser naccarii). Can J Fish Aquat Sci 58:1104–1112
McKenzie DJ, Cataldi E, Taylor EW, Cataudella S, Bronzi P (2001b) Effects of acclimation to brackish water on tolerance of salinity challenge by Adriatic sturgeon (Acipenser naccarii). Can J Fish Aquat Sci 58:1113–1120
McKenzie DJ, Martinez R, Morales A, Acosta J, Morales R, Taylor EW, Steffensen JF, Estrada MP (2003) Effects of growth hormone transgenesis on metabolic rate, exercise performance and hypoxia tolerance in tilapia hybrids. J Fish Biol 63:398–409
Morgan JD, Iwama GK (1991) Effects of salinity on growth, metabolism and ion regulation in juvenile rainbow and steelhead trout (Oncorhynchus mykiss) and fall chinook salmon (Oncorhynchus tshawytscha). Can J Fish Aquat Sci 48:2083–2094
Morgan JD, Iwama GK (1998) Salinity effects on oxygen consumption, gill Na+,K+-ATPase activity and ion regulation in juvenile coho salmon. J Fish Biol 53:1110–1119
Pickett GD. Pawson MG (1994) Seabass. Chapman and Hall, London
Sardella BA, Matey V, Cooper J, Gonzalez RJ, Brauner CJ (2004) Physiological, biochemical and morphological indicators of osmoregulatory stress in ‘California’ Mozambique tilapia (Oreochromis mossambicus x O. urolepis hornorum) exposed to hypersaline water. J Exp Biol 207:1399–1413
Shingles A, McKenzie DJ, Taylor EW, Moretti A, ButlerPJ, Ceradini S (2001) Effects of sublethal ammonia exposure on swimming performance in rainbow trout (Oncorhynchus mykiss). J Exp Biol 204:2691–2698
Swanson C (1998) Interactive effects of salinity on metabolic rate, activity, growth and osmoregulation in the euryhaline milkfish (Chanos chanos). J Exp Biol 201:3355–3366
Taylor SE, Egginton S, Taylor EW (1996) Seasonal temperature acclimatisation of rainbow trout: cardiovascular and morphometric influences on maximum sustainable exercise level. J Exp Biol 199:835–845
Thorarensen H, Gallaugher PE, Farrell AP (1996a) The limitations of heart rate as a predictor of metabolic rate in fish. J Fish Biol 49:226–236
Thorarensen H, Gallaugher PE, Farrell AP (1996b) Cardiac output in swimming rainbow trout, Oncorhynchus mykiss, acclimated to seawater. Physiol Zool 69:139–153
Varsamos S, Connes R, Diaz JP, Barnabé G, Charmantier G (2001) Ontogeny of osmoregulation in the European seabass Dicentrarchus labrax L. Mar Biol 138:909–915
Varsamos S, Diaz JP, Charmantier G, Flik G, Blasco C, Connes R (2002) Branchial chloride cells in seabass (Dicentrarchus labrax) adapted to freshwater, seawater and doubly concentrated seawater. J Exp Zool 293:12–26
Acknowledgements
The authors are grateful to G. Guillou and M. Prineau for their assistance during the study. A.C. was supported by a doctoral bursary provided jointly by the Conseil Régionale Charente Maritime and Ifremer. D.J.M. was employed on a research project funded by the European Commission (Ethofish QLRT-2001-00799). These experiments complied with the current laws in France.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S.A. Poulet, Roscoff
Rights and permissions
About this article
Cite this article
Chatelier, A., McKenzie, D.J. & Claireaux, G. Effects of changes in water salinity upon exercise and cardiac performance in the European seabass (Dicentrarchus labrax). Marine Biology 147, 855–862 (2005). https://doi.org/10.1007/s00227-005-1624-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-1624-7