Abstract
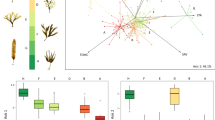
The ability of algae to change the shape of their thallus in response to the environment may be of functional and ecological importance to the alga, with many species of macroalgae exhibiting a great range of morphological variation across wave exposure gradients. However, differences in morphology detected between sheltered and exposed environments cannot determine whether such differences represent plastic responses to the local environment or whether morphology is genetically fixed. This study tested for differences in the morphology of the common kelp, Ecklonia radiata, between wave sheltered and exposed environments, and reciprocally transplanted juveniles to distinguish the nature of such differences (i.e. plastic vs fixed traits). Differences between exposure environments were consistent with known effects of exposure (i.e. a wide, thin thallus at sheltered sites and a narrow, thick thallus with a thick stipe at exposed sites). The reciprocal transplant experiment confirmed that morphological plasticity was the mechanism enabling this alga to display different patterns in morphology between exposure environments. Individuals transplanted to the exposed environment underwent a rapid and extreme response in morphology, which was not apparent in individuals transplanted to the sheltered environment that responded more slowly. These results suggest that stressors typical of sheltered environments (i.e. diffusion stress) may not be as influential (if at all) compared to stressors typical of exposed environments (i.e. breakage, dislodgement) in differentiating morphological characters between exposure environments.





Similar content being viewed by others
References
Agrawal AA (2001) Phenotypic plasticity in the interactions and evolution of species. Science 294:321–326
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust Ecol 26:32–46
Armstrong SL (1989) The behavior in flow of the morphologically variable seaweed Hedophyllum sessile (C. Ag.) Setchell. Hydrobiologia 183:115–122
Baardseth E (1970) A square-scanning, two-stage sampling method of estimating seaweed quantities, vol 20. Report of the Norwegian Institute of Seaweed Research, pp 1–6
Bäck S (1993) Morphological variation of northern Baltic Fucus vesiculosus along the exposure gradient. Annu Bot Fenn 30:275–283
Bell EC, Denny MW (1994) Quantifying ‘wave exposure’:a simple device for recording maximum velocity and results of its use at several field sites. J Exp Mar Biol Ecol 181:9–29
Bertness MD, Gaines SD, Yeh SM (1998) Making mountains out of barnacles: the dynamics of acorn barnacle hummocking. Ecology 79:1382–1394
Blanchette CA (1997) Size and survival of intertidal plants in response to wave action: a case study with Fucus gardneri. Ecology 78:1563–1578
Blanchette CA, Miner BG, Gaines SD (2002) Geographic variability in form, size and survival of Egregia menziesii around Point Conception, California. Mar Ecol Progress Ser 239:69–82
Chapman ARO (1973) Phenetic variability of stipe morphology in relation to season, exposure, and depth in the non-digitate complex of Laminaria Lamour. (Phaeophyta, Laminariales) in Nova Scotia. Phycologia 12:53–57
Chapman ARO (1974) The genetic basis of morphological differentiation in some Laminaria populations. Mar Biol 24:85–91
Cheshire AC, Hallam ND (1988) Morphology of the Southern Bull-Kelp (Durvillaea potatorum, Durvilleales, Phaeophyta) from King Island (Bass Strait, Australia). Bot Mar 31:139–148
Cheshire AC, Hallam ND (1989) Morphological differences in the Southern Bull-Kelp (Durvillaea potatorum) throughout South-Eastern Australia. Bot Mar 32:191–197
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 17:117–143
Cousens R (1982) The effect of exposure to wave action on the morphology and pigmentation of Ascophyllum nodosum (L.) Le Jolis in South-Eastern Canada. Bot Mar 25:191–195
Day RW, Quinn GP (1989) Comparisons of treatments after an analysis of variance in ecology. Ecol Monogr 59:433–463
De Paula EJ, De Oliveira EC (1982) Wave exposure and ecotypical differentiation in Sargassum cymosum (Phaeophyta—Fucales). Phycologia 21:145–153
de Senerpont Domis LN, Fama P, Bartlett AJ, Prud’homme van Reine WF, Armenta Espinosa C, Trono GC (2003) Defining taxon boundaries in members of the morphologically and genetically plastic genus Caulerpa (Caulerpales, Chlorophyta). J Phycol 39:1019–1037
Druehl LD, Kemp L (1982) Morphological and growth responses of geographically isolated Macrocystis integrifolia populations when grown in a common environment. Can J Bot 60:1409–1413
Dudgeon SR, Johnson AS (1992) Thick vs. thin: thallus morphology and tissue mechanics influence differential drag and dislodgement of two co-dominant seaweeds. J Exp Mar Biol Ecol 165:23–43
Dudley SA (1996) The response to differing selection on plant physiological traits: evidence for local adaptation. Evolution 50:103–110
Fowler-Walker MJ, Connell SD, Gillanders BM (2005a) To what extent do geographic and environmental variables correlate with kelp morphology across temperate Australia? Mar Freshw Res 56:877–887
Fowler-Walker MJ, Connell SD, Gillanders BM (2005b) Variation at local scales need not impede tests for broader scale patterns. Mar Biol 147:823–831
Gerard VA, Mann KH (1979) Growth and production of Laminaria longicruris (Phaeophyta) populations exposed to different intensities of water movement. J Phycol 15:33–41
Gerard VA (1982) In situ water motion and nutrient uptake by the giant kelp Macrocystis pyrifera. Mar Biol 69:51–54
Gerard VA (1987) Hydrodynamic streamlining of Laminaria Saccharina Lamour in response to mechanical stress. J Exp Mar Biol Ecol 107:237–244
Goodsell PJ, Fowler-Walker MJ, Gillanders BM, Connell SD (2004) Variations in the configuration of algae in subtidal forests: Implications for invertebrate assemblages. Aust Ecol 29:350–357
Gower JC (1967) A comparison of some methods of cluster analysis. Biometrics 23:623–627
Hanisak MD (1983) The nitrogen relationships of marine macroalgae. In: Carpenter EJ, Capone DG (eds) Nitrogen in the marine environment. Academic, New York, pp 699–730
Hurd CL, Harrison PJ, Druehl LD (1996) Effects of seawater velocity on inorganic nitrogen uptake by morphologically distinct forms of Macrocystis integrifolia from wave-sheltered and exposed sites. Mar Biol 126:205–214
Hurd CL (2000) Water motion, marine macroalgal physiology, and production. J Phycol 36:453–472
Hurlbert SH (1984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54:187–211
Jackelman JJ, Bolton JJ (1990) Form variation and productivity of an intertidal foliose Gigartina species (Rhodophyta) in relation to wave exposure. Hydrobiologia 204/205:57–64
Johnson AS, Koehl MAR (1994) Maintenance of dynamic strain similarity and environmental stress factor in different flow habitats: thallus allometry and material properties of a giant kelp. J Environ Biol 195:381–410
Kalvas A, Kautsky L (1993) Geographic variation in Fucus vesiculosus morphology in the Baltic and North Seas. Eur J Phycol 28:85–91
Kennelly SJ, Underwood AJ (1993) Geographic consistencies of effects of experimental physical disturbance on understorey species in sublittoral kelp forests in central New South Wales. J Exp Mar Biol Ecol 168:35–58
Kirkman H (1981) The first year in the life history and the survival of the juvenile marine macrophytye, Ecklonia radiata (Turn.) J. Agardh. J Exp Mar Biol Ecol 55:243–254
Kirkman H (1984) Standing stock and production of Ecklonia radiata (A.Ag.) J. Agardh. J Exp Mar Biol Ecol 76:119–130
Klinger T, DeWreede RE (1988) Stipe rings, age, and size in populations of Laminaria setchelli Silva (Laminariales, Phaeophyta) in British Columbia, Canada. Phycologia 27:234–240
Koehl MAR (1986) Seaweeds in moving water: Form and mechanical function. In: Gavinish T (ed) On the economy of plant form and function. Cambridge University Press, Cambridge, pp 603–634
Koehl MAR, Alberte RS (1988) Flow, flapping, and photosynthesis of Nereocystis luetkeana: a functional comparison of undulate and flat blade morphologies. Mar Biol 99:435–444
Molloy FJ, Bolton JJ (1996) The effects of wave exposure and depth on the morphology of inshore populations of the Namibian kelp, Laminaria schinzii Foslie. Bot Mar 39:525–531
Norton TA (1969) Growth form and environment in Saccorhiza polyschides. J Mar Biol Ass U K 49:1025–1045
Norton TA, Mathieson AC, Neushul M (1982) A review of some aspects of form and function in seaweeds. Bot Mar 25:501–510
Podani J (1999) Extending Gower’s general coefficient of similarity to ordinal characters. Taxon 48:331–340
Raimondi PT, Forde SE, Delph LF, Lively CM (2000) Processes structuring communities:evidence from trait-mediated indirect effects through induced polymorphisms. Oikos 91:353–361
Ralph PJ, Morrison DA, Addison A (1998) A quantitative study of the patterns of morphological variation within Hormosira banksii (Turner) Decaisne (Fucales: Phaeophyta) in south-eastern Australia. J Exp Mar Biol Ecol 225:285–300
Relyea RA, Werner EE (2000) Morphological plasticity in four larval anurans distributed along an environmental gradient. Copeia 1:178–190
Rice EL, Kenchington TJ, Chapman ARO (1985) Intraspecific geographic-morphological variation patterns in Fucus distichus and Fucus evanescens. Mar Biol 88:207–215
Rice EL, Kenchington TJ (1990) Spatial variation patterns in the marine macroalgal Xiphophora gladiata spp. Gladiata (Phaeophyta). II. Morphological variation over large spatial scales. J Phycol 26:522–534
Roberson LM, Coyer JA (2004) Variation in blade morphology of the kelp Eisenia arborea: incipient speciation due to local water motion? Mar Ecol Prog Ser 282:115–128
Scheiner SM, Goodnight CJ (1984) The comparison of phenotypic plasticity and genetic variation in populations of the grass Danthonia spicata. Evolution 38:845–855
Scott GW, Hull SL, Hornby SE, Hardy FG, Owens NJP (2001) Phenotypic variation in Fucus spiralis (Phaeophyceae): morphology, chemical phenotype and their relationship to the environment. Eur J Phycol 36:43–50
Serisawa Y, Yokohama Y, Aruga Y, Tanaka J (2002) Growth of Ecklonia cava (Laminariales, Phaeophyta) sporophytes transplanted to a locality with different temperature conditions. Phycol Res 50:201–207
Serisawa Y, Aoki M, Hirata T, Bellgrove A, Kurashima A, Tsuchiya Y, Sato T, Ueda H, Yokohama Y (2003) Growth and survival rates of large-type sporophytes of Ecklonia cava transplanted to a growth environment with small-type sporophytes. J Appl Phycol 15:311–318
Slatkin M (1987) Gene flow and the geographic structure of natural populations. Science 236:787–792
Sultan SE (2001) Phenotypic plasticity and ecological breadth in plants. Am Zool 41:1599
Svendsen P, Kain JM (1971) The taxonomic status, distribution, and morphology of Laminaria cucullata sensu Jorde and Klavestad. Sarsia 46:1–21
Topinka JA, Robbins JV (1976) Effects of nitrate and ammonium enrichment on growth and nitrogen physiology in Fucus spiralis. Limnol Oceanogr 21:659–664
Underwood AJ (1997) Experiments in ecology. Their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge, pp 504
Wernberg T, Coleman M, Fairhead A, Miller S, Thomsen M (2003) Morphology of Ecklonia radiata (Phaeophyta: Laminarales) along its geographic distribution in south-western Australia and Australasia. Mar Biol 143:47–55
Wernberg T (2005) Holdfast aggregation in relation to morphology, age, attachment and drag for the kelp Ecklonia radiata. Aquat Bot DOI:10.1016/j.aquabot.2005.04.003
Wheeler WN (1980) Effect of boundary layer transport on the fixation of carbon by the giant kelp Macrocystis pyrifera. Mar Biol 56:103–110
Acknowledgements
This work could not have been completed without the logistical support of B. Russell, S. Hart and D. Gorman. This work was supported by a postgraduate award to M.J.F and an ARC grant to S.D.C.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M.S. Johnson, Crawley
Rights and permissions
About this article
Cite this article
Fowler-Walker, M.J., Wernberg, T. & Connell, S.D. Differences in kelp morphology between wave sheltered and exposed localities: morphologically plastic or fixed traits?. Marine Biology 148, 755–767 (2006). https://doi.org/10.1007/s00227-005-0125-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0125-z