Abstract
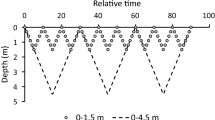
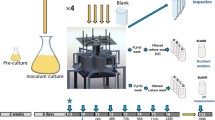
Skeletonema costatum was grown in an outdoor mesocosm to test the hypothesis that fluctuations in irradiance brought about by changes in mixing time and depth can reduce diatom growth and biomass in the turbulent mixed layer. The light environment and mixing regime within the mesocosm were comparable to those in shallow lakes and coastal waters. Experiments showed no significant differences for 24-h mean and 7-day mean chlorophyll a and carbon-specific growth for mixed depths of 1 m and 3 m, and mixing times between 4 min and 65 min. Fluctuations in irradiance brought about by turbulent mixing had no significant effect on specific growth. The relationship between mixed depths and critical depths for S. costatum was therefore independent of fluctuations in irradiance in the turbulent mixed layer. The results indicated that to control growth of S. costatum mixed depths would have to exceed photic depths by a factor of 15, instead of the conventionally accepted factor of 5. Thus, it is likely that artificial mixing of shallow (<10 m) eutrophic waters will be more effective in controlling ‘slow-growing’ summer biomass than ‘fast-growing’ spring blooms dominated by diatoms.








Similar content being viewed by others
References
Bienfang P, Szyper J, Laws E (1983) Sinking rate and pigment responses to light-limitation of a marine diatom: implications to the dynamics of chlorophyll maximum layers. Oceanol Acta 6:55–62
Bienfang PK (1981) SETCOL—a technologically simple and reliable method for measuring phytoplankton sinking rates. Can J Fish Aquat Sci 38:1289–1294
Brand LE, Guillard RRL (1981) The effects of continuous light and light intensity on the reproduction rates of twenty-two species of marine phytoplankton. J Exp Mar Biol Ecol 50:119–132
Clark NN, Dabolt RJ (1986) A general design equation for airlift pumps operating in slug flow. Am Inst Chem Eng J 32:56–64
Cloern JE (1978) Empirical model of Skeletonema costatum photosynthetic rate, with applications to the San Francisco Bay Estuary. Adv Water Resour 1:267–274
Cloern JE (1987) Turbidity as a control on phytoplankton biomass and productivity in estuaries. Cont Shelf Res 7:1367–1381
Cole BE, Cloern JE (1984) Significance of biomass and light availability to phytoplankton productivity in San Francisco Bay. Mar Ecol Prog Ser 17:15–24
Denman KL, Gargett AE (1983) Time and space scales of vertical mixing and advection of phytoplankton in the upper ocean. Limnol Oceanogr 28:801–815
Eppley RW, Coatsworth JL (1966) Culture of the marine phytoplankter, Dunaliella tertiolecta, with light-dark cycles. Arch Mikrobiol 55:66–80
Falkowski PG (1980) Light-shade adaptation in marine phytoplankton. In: Falkowski PG (ed) Primary productivity in the sea. Plenum Press, New York, pp 99–119
Fogg GE, Thake B (1987) Algal cultures and phytoplankton ecology. University of Wisconsin Press, Madison
Foy RH, Gibson CE (1993) The influence of irradiance, photoperiod and temperature on the growth kinetics of three planktonic diatoms. Eur J Phycol 28:203–212
Franco P, Michelato A (1992) Northern Adriatic Sea: oceanography of the basin proper and of the western coastal zone. In: Vollenweider RA, Marchetti R, Viviani R (eds) Marine coastal eutrophication. Elsevier Science, Amsterdam, pp 35–62
Gallagher JC, Wood AM, Alberte RS (1984) Ecotypic differentiation in the marine diatom Skeletonema costatum: influence of light intensity on the photosynthetic apparatus. Mar Biol 82:121–134
Gallegos CL, Platt T (1982) Phytoplankton production and water motion in surface mixed layers. Deep-Sea Res 29:65–76
Gran HH, Braarud T (1935) A quantitative study of the phytoplankton in the Bay of Fundy and the Gulf of Maine (including observations on the hydrography, chemistry and turbidity). Can J Biol Bd 1:279–467
Grobbelaar JU (1989) Do light/dark cycles of medium frequency enhance phytoplankton productivity? J Appl Phycol 1:333–340
Hans M-S, Furuya K, Nemoto T (1992) Species-specific productivity of Skeletonema costatum (Bacillariophyceae) in the inner part of Tokyo Bay. Mar Ecol Prog Ser 79:267–273
Harper D (1992) Eutrophication of freshwaters: principles, problems and restoration. Chapman and Hall, London
Harris GP (1978) Photosynthesis, productivity and growth: the physiological ecology of phytoplankton. Arch Hydrobiol Beih Ergeb Limnol 10:1–171
Harrison PJ, Waters RE, Taylor FJR (1980) A broad spectrum artificial seawater medium for coastal and open ocean phytoplankton. J Phycol 16:28–35
Hitchcock GL, Smayda TJ (1977) The importance of light in the initiation of the 1972–73 winter-spring diatom bloom in Narragansett Bay. Limnol Oceanogr 22:126–131
Hitchcock GL, Goldman JC, Dennett MR (1986) Photosynthate partitioning in cultured marine phytoplankton: metabolic patterns in a marine diatom under constant and variable light intensities. Mar Ecol Prog Ser 30:77–84
Huisman J (1999) Population dynamics of light-limited phytoplankton: microcosm experiments. Ecology 80:202–210
Huisman J, Oostveen P van, Weissing FJ (1999) Critical depth and critical turbulence: two different mechanisms for the development of phytoplankton blooms. Limnol Oceanogr 44:1781–1787
Jorgensen EG, Steeman-Nielsen E (1965) Adaptation in plankton algae. In: Goldman CR (ed) Primary productivity in aquatic environments. University of California Press, Berkeley
Kirk JTO (1994) Light & photosynthesis in aquatic ecosystems. Cambridge University Press, Cambridge
Kromkamp J, Limbeek M (1993) Effect of short-term variation in irradiance on light harvesting and photosynthesis of the marine diatom Skeletonema costatum: a laboratory study simulating vertical mixing. J Gen Microbiol 139:2277–2284
MacIntyre S (1993) Vertical mixing in a shallow, eutrophic lake: possible consequences for the light climate of phytoplankton. Limnol Oceanogr 38:798–817
Marra J (1978) Phytoplankton photosynthetic response to vertical movement in a mixed layer. Mar Biol 46:203–208
Merchuk JC, Siegel MH (1988) Airlift reactors in chemical and biological technology. J Chem Tech Biotechnol 41:105–120
Neale PJ, Heaney SI, Jaworski GHM (1991a) Responses to high irradiance contribute to the decline of the spring diatom maximum. Limnol Oceanogr 36:761–768
Neale PJ, Talling JF, Heaney SI, Reynolds CS, Lund JWG (1991b) Long time series from the English Lake District: irradiance-dependent phytoplankton dynamics during the spring maximum. Limnol Oceanogr 36:751–760
Nicklisch A (1998) Growth and light absorption of some planktonic cyanobacteria, diatoms and chlorophyceae under simulated natural light fluctuations. J Plankton Res 20:105–119
Nixdorf B, Pagenkopf W-G, Behrendt H (1992) Diurnal patterns of mixing depth and its influence on primary production in a shallow lake. Int Rev Ges Hydrobiol 77:349–360
Obata A, Ishizaka J, Endoh M (1996) Global verification of critical depth theory for phytoplankton bloom with climatological in situ temperature and satellite ocean color data. J Geophys Res 101:20657–20667
Parsons TR, Maita Y, Lali CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, Oxford
Parsons TR, Takahashi M, Hargrave B (1990) Biological oceanographic processes. Pergamon Press, Oxford
Petersen JE, Chen C, Kemp WM (1997) Scaling aquatic primary productivity: experiments under nutrient- and light-limited conditions. Ecology 78:2326–2338
Petersen JE, Sanford LP, Kemp WM (1998) Coastal plankton responses to turbulent mixing in experimental ecosystems. Mar Ecol Prog Ser 171:23–41
Qiang H, Richmond A (1996) Productivity and photosynthetic efficiency of Spirulina platensis as affected by light intensity, algal density and rate of mixing in a flat plate photobioreactor. J Appl Phycol 8:139–145
Queen Mary & Westfield College (1992) Laboratory work. In: An ecosystem approach to understanding pollutant inputs and algal bloom and mucilage problems in the Adriatic Sea. (EC STEP CT90 0061 second six monthly report) Environmental Research & Development Programme, Commission of the European Communities
Queen Mary & Westfield College (1994) Field monitoring and measurements of productivity. In: An ecosystem approach to understanding pollutant inputs and algal bloom and mucilage problems in the Adriatic Sea. (EC STEP CT90 0061 final report) Environmental Research & Development Programme, Commission of the European Communities
Revelante N, Gilmartin M (1980) Microplankton diversity indices as indicators of eutrophication in the northern Adriatic Sea. Hydrobiologia 70:277–286
Reynolds CS (1990) Temporal scales of variability in pelagic environments and the response of phytoplankton. Freshw Biol 23:25–53
Reynolds CS (1993) Swings and roundabouts: engineering the environment of algal growth. In: White KN, Bellinger EG, Saul AJ, Symes M, Hendry K (eds) Urban waterside regeneration, problems & prospects. Ellis Horwood, Chichester
Reynolds CS, Wiseman SW, Clarke MJO (1984) Growth and loss-rate responses of phytoplankton to intermittent artificial mixing and their application to the control of planktonic algal biomass. J Appl Ecol 21:11–39
Riddle AM, Lewis RE (2000) Dispersion experiments in U.K. coastal waters. Estuar Coast Shelf Sci 51:243–254
Riley GA (1957) Phytoplankton of the north central Sargasso Sea, 1950–1952. Limnol Oceanogr 2:252–270
Sakshaug E, Andresen K (1986) Effect of light regime upon growth rate and chemical composition of a clone of Skeletonema costatum from the Trondheimsfjord, Norway. J Plankton Res 8:619–637
Sanford LP (1997) Turbulent mixing in experimental ecosystem studies. Mar Ecol Prog Ser 161:265–293
Savidge G (1986) Growth and photosynthetic rates of Phaeodactylum tricornutum Bohlin in a cyclical light field. J Exp Mar Biol Ecol 100:147–164
Siegel DA, Doney SC, Yoder JA (2002) The North Atlantic spring phytoplankton bloom and Sverdrup’s critical depth hypothesis. Science 296:730–733
Steinberg CEW, Gruhl E (1992) Physical measures to inhibit planktonic cyanobacteria. In: Sutcliffe DW, Jones JG (eds) Eutrophication: research and application to water supply. (Special publication no. 3) Freshwater Biological Association, Ambleside, Cumbria, UK
Sverdrup HU (1953) On conditions for the vernal blooming of phytoplankton. J Cons Perm Int Explor Mer 18:287–295
Talling JF (1955) The relative growth rates of three plankton diatoms in relation to underwater radiation and temperature. Ann Bot 19:329–341
Talling JF (1971) The underwater light climate as a controlling factor in the production ecology of freshwater phytoplankton. Mitt Int Verein Limnol 19:214–243
Thornton DCO, Thake B (1998) Effect of temperature on the aggregation of Skeletonema costatum (Bacillariophyceae) and the implication for carbon flux in coastal waters. Mar Ecol Prog Ser 174:223–231
Townsend DW, Keller MD, Sieracki ME, Ackleson SG (1992) Spring phytoplankton blooms in the absence of vertical water column stratification. Nature 360:59–62
Vollenweider RA, Rinaldi A, Montanari G (1992) Eutrophication, structure and dynamics of a marine coastal system: results of ten-year monitoring along the Emilia-Romagna coast (northwest Adriatic Sea). In: Vollenweider RA, Marchetti R, Viviani R (eds) Marine coastal eutrophication. Elsevier Science, Amsterdam
Walsby AE, Reynolds CS (1980) Sinking and floating. In: Morris I (ed) The physiological ecology of phytoplankton. (Studies in ecology, 7) Blackwell Scientific, Oxford
Wellnitz T, Rinne B (1999) Photosynthetic response of stream periphyton to fluctuating light regimes. J Phycol 35:667–672
Zevenboom W, Mur LR (1984) Growth and photsynthetic response of the cyanobacterium Microcystis aeruginosa in relation to photoperiodicity and irradiance. Arch Microbiol 139:232–239
Acknowledgements
We thank the Royal Commission for the Exhibition of 1851 for an award to Dipen Patel that made this research possible. We are most grateful to Mr. Bob Head at the Plymouth Marine Laboratory, England, for his kind assistance in analysing the carbon samples. The experiments described comply fully with current United Kingdom laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P.W. Sammarco, Chauvin
Rights and permissions
About this article
Cite this article
Patel, D., Thake, B. & Thornton, D.C.O. Effect of light and turbulent mixing on the growth of Skeletonema costatum (Bacillariophyceae). Marine Biology 146, 633–644 (2005). https://doi.org/10.1007/s00227-004-1486-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-004-1486-4