Abstract
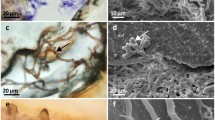
Endolithic fungi bore through the extracellular calcium carbonate skeleton of reef-building scleractinian corals, both healthy and dead, and effect net erosion of coral reefs. Potential fungal interactions with coral tissue were investigated using an in vitro approach suggested by earlier observations of skeletal repair cones at the site of fungal perforation in Porites sp. A fungal strain was isolated from the skeleton of a long-term culture of healthy, tissue-covered, Pocillopora damicornis Linnaeus colonies maintained in a recirculating system in Monaco. As coral soft tissue spontaneously dissociated in vitro, the skeleton became exposed and hyaline hyphae emerged radially from 15% of the total clipped branches. In this study, which was performed between January 2001 and March 2003, 35 skeleton–hypha explants were embedded in agar-based solid medium, yielding 60% hyphal growth. A fungal strain (F19-3-1) of the dominant (80%) morphology was isolated and propagated in agar-based solid medium. The strain was identified by 18S and 26S rDNA gene sequence analysis as a basidiomycete in the genus Cryptococcus. Co-cultures were used to provide experimental exposure of coral soft tissue to the fungus. The fungus extended the survival of coral cells by 2 days, selectively maintaining skeletogenic cell types. This effect may be interpreted as stimulation by the fungus of a short-term coral defense response.





Similar content being viewed by others
References
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bentis CJ, Kaufman L, Golubic S (2000) Endolithic fungi in reef-building corals (order Scleractinia) are common, cosmopolitan and potentially pathogenic. Biol Bull (Woods Hole) 198:254–260
Boekhout T, Bandoni RJ, Fell JW, Kwon-Chung KJ (1998) Discussion of teleomorphic and anamorphic genera of heterobasidiomycetous yeasts. In: Kurtzman CP, Fell JW (eds) The yeasts: a taxonomic study. Elsevier, Amsterdam, pp 609–626
Borneman J, Hartin RJ (2000) PCR primers that amplify fungal rRNA genes from environmental samples. Appl Environ Microbiol 66:4356–4360
Chun J (1995) Computer-assisted classification and identification of actinomycetes. PhD thesis, University of Newcastle upon Tyne, Newcastle upon Tyne
Colas V, Conrod S, Venard P, Keller H, Ricci P, Panabières F (2001) Elicitin genes expressed in vitro by certain tobacco isolates of Phytophthora parasitica are down regulated during compatible interactions. Mol Plant-Microbe Interact 14:326–335
Cuif J-P, Dauphin Y (1998) Microstructural and physico-chemical characterization of ‘centers of calcification’ in septa of some recent scleractinian corals. Palaeontologische Z 72:257–270
Domart-Coulon I, Elbert DC, Scully EP, Calimlim PS, Ostrander GK (2001) Aragonite crystallization in primary cell cultures of multicellular isolates from a hard coral Pocillopora damicornis. Proc Natl Acad Sci USA 98:11885–11890
Fell JW, Newell SY (1998) Biochemical and molecular methods for the study of marine fungi. In: Cooksey KE (ed) Molecular approaches to the study of the ocean. Chapman and Hall, London, pp 259–283
Fell JW, Boekhout T, Fonseca A, Scorzetti G, Statzell-Tallman A (2000) Biodiversity and systemics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int J Syst Evol Microbiol 50:1351–1371
Felsenstein J (1993) PHYLIP (Phylogenetic Inference Package), version 3.5c. Department of Genetics, University of Washington, Seattle
Fitch WM, Margoliash E (1967) Construction of phylogenetic trees: a method based on mutation distances as estimated from cytochrome c sequences is of general applicability. Science 155:279–284
Frank U, Rabinowitz C, Rinkevich B (1994) In vitro establishment of continuous cell cultures and cell lines from ten colonial cnidarians. Mar Biol 120:491–499
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HN (ed) Mammalian protein metabolism. Academic, New York, pp 21–132
Kendrick B, Risk MJ, Michaelides J, Bergman K (1982) Amphibious microborers: bioeroding fungi isolated from live corals. Bull Mar Sci 32:862–867
Kluge AG, Farris FS (1969) Quantitative phyletics and the evolution of anurans. Syst Zool 18:1–32
Kohlmeyer J (1969) The role of marine fungi in the penetration of calcareous substances. Am Zool 9:741–746
Kohlmeyer J, Volkmann-Kohlmeyer B (1987) Koralionastetaceae fam. nov. (ascomycetes) from coral rock. Mycologia 79:764–778
Kopecky EJ, Ostrander GK (1999) Isolation and primary culture of viable multicellular endothelial isolates from hard corals. In Vitro Cell Dev Biol Anim 35:616–624
Le Campion-Alsumard T, Golubic S, Priess K (1995) Fungi in corals: symbiosis or disease? Interaction between polyps and fungi causes pearl-like skeleton biomineralization. Mar Ecol Prog Ser 117:137–147
Maidak BL, Cole JR, Parke CT Jr, Garrity GM, Larsen N, Li B, Lilburn TG, McCaughey MJ, Olsen GJ, Overbeek R, Pramanik S, Schmidt TM, Tiedje JM, Woese CR (1999) A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res 27:171–173
Marubini F, Davies PS (1996) Nitrate increases zooxanthellae population density and reduces skeletogenesis in corals. Mar Biol 127:319–328
Priess K, Le Campion-Alsumard T, Golubic S, Gadel F, Thomassin BA (2000) Fungi in corals: black bands and density-banding of Porites lutea and P. lobata skeleton. Mar Biol 136:19–27
Puverel S, Tambutté E, Bouchot A, Zoccola D, Payan P, Cuif JP, Allemand D (2003) The organic matrix of the scleractinian coral Stylophora pistillata. In: Proceedings of the 8th international symposium on biomineralization “biomineralization: formation, diversity, evolution and application” 25–28 September 2001. Kurikawa, Niigata, Japan (in press)
Ravindran J, Raghukumar C, Raghukumar S (2001) Fungi in Porites lutea: association with healthy and diseased corals. Dis Aquat Org 47:219–228
Ricci P (1997) Induction of the hypersensitive response and systemic acquired resistance by fungal proteins: the case of elicitins. In: Stacey G, Keen NT (eds) Plant–Microbe interactions, vol 3. Chapman and Hall, New York, pp 53–75
Rozen S, Skaletsky HJ (2000) Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S (eds) Bioinformatics methods and protocols: methods in molecular biology. Humana, Totowa, N.J., pp 365–386
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Smith GW, Ives LD, Nagelkerken IA, Ritchie KB (1996) Caribbean sea-fan mortalities. Nature 383:487
Sreenivasaprasad S (2000) Isolation of fungal nucleic acids. In: Rapley R (ed) The nucleic acid protocols handbook. Humana, Totowa, N.J., pp 37–45
Tribollet A, Decherf G, Hutchings PA, Peyrot-Clausade M (2002) Large scale spatial variability in bioerosion of experimental coral substrates on the Great Barrier Reef (Australia): importance of microborers. Coral Reefs 21:424–432
Wainwright S (1963) Skeletal organization in the coral Pocillopora damicornis. Q J Micrsco Sci 104:169–183
Acknowledgements
This work was supported by the Centre Scientifique de Monaco (CSM) (postdoctoral fellowship to I. Domart-Coulon and doctoral fellowship to S. Puverel). The authors wish to thank aquariologists D. Desgres and C. Richard (CSM) for providing coral, J.M. McCaffery (Johns Hopkins University) for assistance with transmission electron microscopy, and E. Tambutté (CSM) and P. Gounon (Université Nice Sophia Antipolis) for assistance with scanning electron microscopy. We thank E. Scully (Towson University) for early discussions leading to this research. We are grateful to Dr. D. Allemand for comments and to Dr. T. Le Campion-Alsumard for encouragement on the work in progress. This is contribution no. 03-591 from the Center of Marine Biotechnology. The experiments reported in this manuscript comply with the current laws in the Principality of Monaco and the United States of America.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Domart-Coulon, I.J., Sinclair, C.S., Hill, R.T. et al. A basidiomycete isolated from the skeleton of Pocillopora damicornis (Scleractinia) selectively stimulates short-term survival of coral skeletogenic cells. Marine Biology 144, 583–592 (2004). https://doi.org/10.1007/s00227-003-1227-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1227-0