Abstract
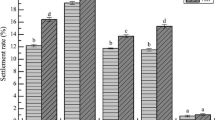
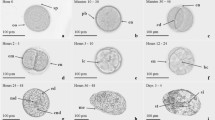
In Japan, mass-production of sea urchin juveniles involves the culture of periphytic diatom films on plastic plates in 5- to 15-tonne tanks for the induction of larval metamorphosis. This study focused on the larval response of sea urchins, Pseudocentrotus depressus and Anthocidaris crassispina, to natural microbial films in the sea and diatom-based films formed in the tanks. The effect of diatoms and bacteria on larval metamorphosis was also examined using laboratory-cultured diatom-based films in the presence of germanium dioxide and antibiotics during film culture. Furthermore, the nature of the cue of the cultured diatom-based film was also investigated. Results showed that P. depressus and A. crassispina metamorphosed both on natural microbial films and diatom-based films in a tank. In the sea, the metamorphosis (%) of P. depressus increased gradually in accordance with the immersion period of film formed on glass slides, whereas the larval metamorphosis of A. crassispina had a bell-shaped response curve. In the tank, although the diatom-based films showed a low inducing activity for larval metamorphosis of A. crassispina, the metamorphosis of P. depressus larvae increased linearly in accordance with the diatom density. These results suggest that diatom-based films could promote the larval metamorphosis of P. depressus, but are less important in A. crassispina. In a simultaneous larval assay (May), P. depressus showed a higher percentage of metamorphosis than A. crassispina. We concluded that the former is more sensitive to diatom-based film than the latter and that this is due to differences in their natural habitats. For laboratory-cultured diatom-based film, both species of sea urchins showed a similar response, in which reduction in diatom and bacteria density resulted in a decrease in the original inducing activity. There seems to be a synergistic effect between diatom and bacteria in inducing larval metamorphosis. Films subjected to treatment with 0.1 N HCl were no longer inductive for either sea urchin, while those films treated with 40°C heat or EtOH (5% and 10% EtOH) showed a significant reduction in the inducing activity. Thus the surface-associated cues may be highly susceptible to the above treatments.





Similar content being viewed by others
References
Barker MF (1977) Observation on the settlement of the branchiolaria larvae of Stichaster australis (Verrill) and Coscinasterias calamaria (Gray) (Echinodermata: Asteroidea) in the laboratory and on the shore. J Exp Mar Biol Ecol 30:95–108
Barker MF, Nichols D (1983) Reproduction, recruitment and juvenile ecology of the starfish, Asterias rubens and Marthasterias glacialis. J Mar Biol Assoc UK 63:745–765
Bhosle NB, Wagh AB (1997) Amino acids in biofilm material on aluminium panels immersed in marine waters. Biofouling 11:149–166
Brancato MS, Woollacott RM (1982) Effects of microbial films on settlement of bryozoan larvae (Bugula simplex, B. stolonifera and B. turrita). Mar Biol 71:51–56
Cameron RA, Hinegardner RT (1974) Initiation of metamorphosis in laboratory cultured sea urchins. Biol Bull 146:335–342
Cameron RA, Schroeter SC (1980) Sea urchin recruitment: effect of substrate selection on juvenile distribution. Mar Ecol Prog Ser 2:243–247
Chen CP, Run JQ (1989) Larval growth and bacteria-induced metamorphosis of Arachnoides placenta (L.) (Echinodermata: Echinoidea). In: Ryland JS, Tyler PA (eds) Reproduction, genetics and distributions of marine organisms. Olsen & Olsen, Fredensborg, Denmark, pp 55-59
Crisp DJ (1974) Factors influencing settlement of marine invertebrate larvae. In: Grant PT, Mackie AM (eds) Chemoreception in marine organisms. Academic Press, London, pp 177–265
Crisp DJ, Walker G, Young GA, Yule AB (1985) Adhesion and substrate choice in mussels and barnacles. J Colloid Interface Sci 104:40–50
Imai T (1980a) On the sea urchins off Miura city. The study of distribution, environment, growth and gonad in Jogashima (in Japanese). Bull Kanagawa Pref Exp Stn 1:35–79
Imai T (1980b) On the sea urchins off Miura city. The study of distribution, environment, growth and gonad from Hatsuse to Kamimiyata (in Japanese). Bull Kanagawa Pref Exp Stn 2:27–36
Imai T, Arai S (1994) Local peculiarities as living for the red sea urchin, Pseudocentrotus depressus of bishamon waters off southern Miura Peninsula, Kanagawa Prefecture, Japan (in Japanese with English abstract). Suisanzoshoku 42:307–313
Ito Y (1984) The effect of benthic diatoms on the acceleration of metamorphosis of sea urchin larva (in Japanese). Mar Fouling 5:15–18
Ito S, Kitamura H (1997) Induction of larval metamorphosis in the sea cucumber, Stichopus japonicus by periphytic diatoms. Hydrobiologia 358:281–284
Ito S, Kobayakawa J, Tani Y, Nakamura N (1991) The combined effect of Hizikia fusiformis with attaching diatoms for larval settlement and metamorphosis of Hemicentrotus pulcherrimus and Pseudocentrotus depressus (in Japanese). Saibaigiken 19:61–66
Johnson CR, Sutton DC (1994) Bacteria on the surface of crustose coralline algae induce the metamorphosis of crown-of-thorns starfish. Mar Biol 120:305–310
Johnson CR, Sutton DC, Olson RR, Giddins R (1991a) Settlement of crown-of-thorns starfish: role of bacteria on surfaces of coralline algae and a hypothesis for deepwater recruitment. Mar Ecol Prog Ser 71:143–162
Johnson CR, Muir DG, Reysenbach AL (1991b) Characteristic bacteria associated with surfaces of coralline red algae: a hypothesis for bacterial induction of marine invertebrate larvae. Mar Ecol Prog Ser 74:281–294
Johnson CR, Lewis TE, Nichols DS, Degnan BM (1997) Bacterial induction of settlement and metamorphosis in marine invertebrates. Proc 8th Int Coral Reef Symp 2:1219–1224
Kawamura T (1996) The role of benthic diatoms in the early life stages of the Japanese abalone (Haliotis discus hannai). In: Watanabe Y, Yamashita Y, Oozeki Y (eds) Survival strategies in early life stages of marine resources. Balkema, Rotterdam, pp 355–367
Kawamura T, Kikuchi S (1992) Effect of benthic diatoms on settlement and metamorphosis of abalone larvae (in Japanese). Suisan-Zoshoku 40:403–409
Keough MJ, Raimondi PT (1995) Responses of settling invertebrate larvae to bio-organic films: effects of different types of films. J Exp Mar Biol Ecol 185:235–253
Kirchman D, Graham S, Reish D, Mitchell R (1982) Bacteria induce settlement and metamorphosis of Janua (Dexiospira) brasiliensis Grube (Polychaeta: Spirobidae). J Exp Mar Biol Ecol 56:153–163
Kitamura H, Hirayama K (1984) Suitable food plankton for growth of a bryozoan Bugula neritina under laboratory conditions. Bull Jpn Soc Sci Fish 50: 973–977
Kitamura H, Kitahara S, Koh HB (1993) The induction of larval settlement and metamorphosis of two sea urchins, Pseudocentrotus depressus and Anthocidaris crassispina, by free fatty acids extracted from the coralline red algae Corallina pilulifera. Mar Biol 115:387–392
Kitamura H, Ito S, Tominaga S (2000) Relationship between the density of benthic diatoms and the amount of chlorophyll-a on plastic plates in sea urchin and sea cucumber tanks (in Japanese). Sessile Organisms 16:21–25
Koh HB, Tsuchida T, Kitamura H, Hirayama K (1996) Effect of dibromomethane on the larval metamorphosis of the sea urchins, Anthocidaris crassispina and Pseudocentrotus depressus (in Japanese). Sessile Organisms 13: 7–9
Lau SCK, Qian PY (2001) Larval settlement in the serpulid polychaete Hydroides elegans in response to bacterial films: an investigation of the nature of putative larval settlement cue. Mar Biol 138:321–328
Leitz T, Wagner T (1993) The marine bacterium Alteromonas espejiana induces metamorphosis of the hydroid Hydractinia echinata. Mar Biol 115:173–178
Loeb GI, Neihof RA (1975) Marine conditioning film. Adv Chem Ser 145:319–335
Maki JS, Rittschof D, Costlow JD, Mitchell R (1988) Inhibition of attachment of larval barnacles, Balanus amphitrite, by bacterial surface films. Mar Biol 97:199–206
Mercier A, Battaglene SC, Hamel J-F (2000) Settlement preferences and early migration of the tropical sea cucumber Holothuria scabra. J Exp Mar Biol Ecol 249:89–110
Mitchell R, Kirchman D (1984) The microbial ecology of marine surfaces. In: Costlow JD, Tipper RC (eds) Marine biodeterioration: an interdisciplinary study. Naval Institutes Press, Annapolis, Md., pp 49–56
Pawlik JR (1992) Chemical ecology of the settlement of benthic marine invertebrates. Oceanogr Mar Biol Annu Rev 30:273–335
Pearce CM, Scheibling RE (1991) Effect of macroalgae, microbial films, and conspecifics on the induction of metamorphosis of the green sea urchin Strongylocentrotus droebachiensis (Müller). J Exp Mar Biol Ecol 147:147–162
Pechenik JA, Rittschoff D, Schmidt AR (1993) Influence of delayed metamorphosis on survival and growth of juvenile barnacles Balanus amphitrite. Mar Biol 115:287–294
Robert RD, Nicholson CM (1997) Variable response from abalone larvae (Haliotis iris, H. virginea) to a range of settlement cues. Molluscan Res 18:131–141
Rowley RJ (1989) Settlement and recruitment of sea urchins (Strongylocentrotus spp.) in a sea urchin barren ground and kelp bed: are populations regulated by settlement or post-settlement processes? Mar Biol 100:485–494
Satuito CG, Natoyama K, Yamazaki M, Fusetani N (1995) Induction of attachment and metamorphosis of laboratory cultured mussel Mytilus edulis, M. galloprovincialis larvae by microbial film. Fish Sci 61:223–227
Slattery M (1992) Larval settlement and juvenile survival in the red abalone (Haliotis rufescens): an examination of inductive cues and substrate selection. Aquaculture 102:143–153
Snelgrove PVR, Grassle JP, Grassle JF, Petrecca RF, Ma H (1999) Notes: In situ habitat selection by settling larvae of marine soft-sediment invertebrates. Limnol Oceanogr 44:1341–1347
Takahashi Y, Itoh K, Ishii M, Suzuki M, Itabashi Y (2002) Induction of larval settlement and metamorphosis of the sea urchin Strongylocentrotus intermedius by glycoglycerolipids from the green alga Ulvella lens. Mar Biol 140:763–771
Tani Y, Ito Y (1979) Effects of benthic diatoms on settlement and metamorphosis of the sea urchin, Pseudocentrotus depressus (in Japanese with English abstract). Suisanzoshoku 27:148–150
Taniguchi K, Kurata K, Maruzoi T, Suzuki M (1994) Dibromomethane, a chemical inducer of settlement and metamorphosis of the sea urchin larvae. Fish Sci 60:795–796
Tsuji S, Yoshiya M, Tanaka M, Kuwahara A, Uchino K (1989) Seasonal changes in distribution and ripeness of gonad of a sea urchin Strongylocentrotus nudus in the western part of Wakasa Bay (in Japanese). Bull Kyoto Ocean Fish Sci 12:15–21
Tsurumi K, Fusetani N (1998) Effects of early fouling communities formed in the field on settlement and metamorphosis of cyprids of the barnacle, Balanus amphitrite Darwin. Biofouling 12:119–131
Unabia CRC, Hadfield MG (1999) Role of bacteria in larval settlement and metamorphosis of the polychaete Hydroides elegans. Mar Biol 133:55–64
Wieczorek SK, Todd CD (1998) Inhibition and facilitation of settlement of epifaunal marine invertebrate larvae by microbial biofilm cues. Biofouling 12:81–118
Williamson JE, Nys RD, Kumar N, Carson DG, Steinberg PD (2000) Induction of metamorphosis in sea urchin Holopneustes purpurascens by a metabolites complex from the algal host Delisea pulchra. Biol Bull 198:332–345
Zobell CE, Allen EC (1935) The significance of marine bacteria in the fouling of submerged surfaces. J Bacteriol 29:239–251
Acknowledgements
The authors are grateful to the staff of the fish farming centers in Saga and Nagasaki City for providing us with sea urchin larvae and for making their facilities available. The first author acknowledges the Ministry of Education, Science, Sports and Culture of Japan and the Malaysian government for providing a fellowship during the study period.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Ikeda, Hakodate
Rights and permissions
About this article
Cite this article
Rahim, S.A.K.A., Li, JY. & Kitamura, H. Larval metamorphosis of the sea urchins, Pseudocentrotus depressus and Anthocidaris crassispina in response to microbial films. Marine Biology 144, 71–78 (2004). https://doi.org/10.1007/s00227-003-1171-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1171-z