Abstract
There is increasing evidence that suspension feeders play a significant role in plankton–benthos coupling. However, to date, active suspension feeders have been the main focus of research, while passive suspension feeders have received less attention. To increase our understanding of energy fluxes in temperate marine ecosystems, we have examined the temporal variability in zooplankton prey capture of the ubiquitous Mediterranean gorgonian Leptogorgia sarmentosa. Prey capture was assessed on the basis of gut content from colonies collected every 2 weeks over a year. The digestion time of zooplankton prey was examined over the temperature range of the species at the study site. The main prey items captured were small (80–200 µm), low-motile zooplankton (i.e. eggs and invertebrate larvae). The digestion time of zooplankton prey increased when temperature decreased (about 150% from 21°C to 13°C; 15 h at 13°C, 9 h at 17°C, and 6 h at 21°C), a pattern which has not previously been documented in anthozoans. Zooplankton capture rate (prey polyp−1 h−1) varied among seasons, with the greatest rates observed in spring (0.16±0.02 prey polyp−1 h−1). Ingestion rate in terms of biomass (μg C polyp−1 h−1) showed a similar trend, but the differences among the seasons were attenuated by seasonal differences in prey size. Therefore, ingestion rate did not significantly vary over the annual cycle and averaged 0.019±0.002 μg C polyp−1 h−1. At the estimated ingestion rates, the population of L. sarmentosa removed between 2.3 and 16.8 mg C m−2 day−1 from the adjacent water column. This observation indicates that predation by macroinvertebrates on seston should be considered in energy transfer processes in littoral areas, since even species with a low abundance may have a detectable impact.
Similar content being viewed by others
Introduction
Quantification of energy and matter transfer processes between the various components of the ecosystem is one of the most complex aspects of marine ecology. Although energy and matter exchanges between plankton and benthos are crucial to our understanding of littoral ecosystems, the contribution of benthic suspension feeders to the rate of these exchanges has only recently been coming to light (Gili and Coma 1998). In shallow areas, active filter feeders may affect plankton communities in the water column by significantly reducing plankton abundance under certain conditions (e.g. Cloern 1982; Officer et al. 1982; Fréchette et al. 1989; Kimmerer et al. 1994; Riisgård et al. 1998). There is increasing evidence that passive suspension feeders may also play a significant role in plankton–benthos coupling (e.g. Coma et al. 1994; Gili et al. 1998). However, research has focused mainly on active suspension feeders and less attention has been devoted to the impact of passive feeders.
Studies on the effect of benthic suspension feeders on plankton abundance in littoral ecosystems have focused mainly on phytoplankton (e.g. Cloern 1982; Fréchette et al. 1989; Riisgård et al. 1998). Zooplankton is a major food source for taxa such as anthozoans and hydrozoans (e.g. Lewis 1982; Sebens and Koehl 1984; Coma et al. 1995), and also for other taxa that until recently have been regarded as microphagous filter feeders, such as bivalves and ascidians (Bingham and Walters 1989; Davenport et al. 2000). Nevertheless, fewer studies have focused on the impact of benthic suspension feeders on zooplankton than on phytoplankton.
Knowledge of the temporal variability of prey capture rates (i.e. annual and inter-annual variation) is essential to understand ecosystem energy fluxes. In the present study we examined temporal variability over the annual cycle. In cold and temperate marine ecosystems, the high degree of environmental variability throughout the year has a major impact on biological processes. Temperature and food availability are factors that are strongly affected by this variability and are crucial in the determination of temporal variability in the prey capture of benthic suspension feeders (Graf et al. 1983; Clarke 1988; Coma et al. 2000). In temperate seas, zooplankton density usually exhibits a marked seasonal pattern (e.g. Siokou-Frangou 1996). Together with variation in hydrodynamic processes, this seasonal pattern may strongly affect the availability of resources to benthic suspension feeders (Coma and Ribes 2003). The zooplankton capture rate of benthic suspension feeders has generally been determined by means of gut content examinations over a short time period (e.g. Porter 1974; Lasker 1981; Sebens and Koehl 1984; Coma et al. 1999). In temperate seas, sampling throughout the annual cycle is a fundamental step in examining temporal variability in prey capture rates. However, because of logistical constraints, there are few studies that sample over a year.
Temperature variation throughout the annual cycle may strongly affect prey digestion time, which is a crucial element in estimating prey capture rate on the basis of gut content examinations. Some laboratory studies of prey digestion time for passive benthic suspension feeders have been conducted (Kinne and Paffenhöfer 1965; Paffenhöffer 1968), but few field approaches have yet been developed (Coffroth 1984; Coma et al. 1994). Furthermore, despite the importance of prey digestion time in estimating prey capture rate, few studies have examined the effect of temperature on prey digestion time for benthic suspension feeders (but see Kinne and Paffenhöfer 1965).
Gorgonians are conspicuous components of littoral benthic ecosystems (True 1970; Kinzie 1973; Starmans et al. 1999). The three-dimensional structure of gorgonians allow them to escape in size from the mainly two-dimensional plane of most benthic communities, thereby favoring the interaction of their capture structures with available seston. Gorgonians contribute significantly to providing habitats for epifauna composed of small species and to increasing the biomass and diversity of the community (Wendt et al. 1985; Mitchell et al. 1992). Therefore, gorgonian communities may play a significant role in the plankton–benthos trophic interaction, and hence in the matter transfer processes between the two systems in littoral ecosystems. Examination of the feeding rate of these communities is the first step in evaluating the significance of their role in matter and energy transfer processes in these ecosystems.
In this study we examined temporal variability in the zooplankton prey capture rate of the ubiquitous Mediterranean gorgonian Leptogorgia sarmentosa (Cnidaria: Octocorallia), which is found in benthic communities usually dominated by boulders and organic debris (Pêrès 1967). Temporal variability in the zooplankton prey capture rate was studied by means of a field survey that examined the annual variation in prey abundance and gut contents, field experiments on prey digestion time, and laboratory analyses that estimated gut content, prey abundance and biomass of the gorgonian species. Prey digestion time was examined over the natural range of temperatures for the species at the study site. The study was concepted to estimate feeding of L. sarmentosa on zooplankton, to determine the effect of temperature on prey digestion time and to evaluate the consequences of this effect on estimates of ingesta over the annual cycle. In addition, by using the estimates of zooplankton feeding and prey digestion times together with data from previous feeding studies of the species on other seston fractions (Ribes et al. 2003), the study elucidates the role of passive suspension feeders in energy transfer processes in temperate littoral ecosystems.
Materials and methods
The Leptogorgia sarmentosa population studied was located at the Tascó Gran rock in the Medes Islands Marine Reserve (NW Mediterranean Sea; 42°3′N; 3°13′E). Colonies are found on small stones at a depth of about 20 m. Population density at the study site was 1.5 colonies m−2; a density within the natural range of those previously reported for the species (Weinberg 1978; Mistri 1995).
Feeding on zooplankton
Feeding on zooplankton was assessed by means of gut content examinations of apical fragments. L. sarmentosa colonies were sampled at approximately 2-week intervals from 11 August 1997 to 27 August 1998. All samples were collected during the same time period (9–11 h) to preclude possible circadian influences on the annual pattern of prey capture. Each sample consisted of one apical fragment collected from five randomly selected colonies. The fragments were immediately placed in 10% formaldehyde solution in seawater to prevent further digestion. The contents of 50 polyps selected at random from each sample (ten from each apical fragment) were isolated by dissection under a binocular microscope, identified to the higher taxon level and counted. The length of all prey was measured under the microscope.
Zooplankton density
Two zooplankton samples were collected concurrently with the collection of fragments of L. sarmentosa colonies for the study of annual variation of plankton. Plankton nets, 22 cm in diameter with a mesh size of 100 μm, were used. The nets were towed over a distance of 40 m by a SCUBA diver, a short distance (30–50 cm) from the gorgonian community. The zooplankton was immediately placed in 4% formaldehyde solution in seawater and was then identified to the level of main taxonomic groups and counted. Pearson’s product-moment correlation (Sokal and Rohlf 1995) was used to examine the relationship between zooplankton prey capture rate and abundance of zooplankton.
Prey digestion time
Three experiments were carried out at a range of temperatures to estimate prey digestion time. The experiments were conducted on 13 August 1999 (21°C), 26 June 1999 (17°C) and 26 February 2000 (13°C), to cover the natural temperature range of the species at the study site (12–22°C). For each experiment, one apical fragment was randomly collected (i.e. cut off) from between 40 and 55 colonies. After collection, five of the fragments were randomly selected and preserved (10% formaldehyde solution in seawater). Each of the remaining branch tips was then attached to a PVC post (1 cm in diameter, 1 cm tall) embedded in a small cement base (3 cm in diameter, 1 cm tall). Previous experiments with this technique showed that transplanted branches behave as ambient conspecifics shortly after the manipulation (Kim and Lasker 1997; Ribes et al. 1998, 1999). The transplanted branches were placed in a container with aerated, filtered (GF/F glass fiber filters) seawater at the natural seawater temperature to prevent further prey capture. The container was placed in shallow water so that ambient seawater temperature was maintained during the experiment. At 1-h intervals, five additional fragments were randomly selected and preserved. The experiment lasted 7 h at 21°C, 7 h at 17°C and 11 h at 13°C. The contents of 50 polyps selected at random from each sample (ten from each apical fragment) were examined in the same manner as the field collections.
Prey capture rate
The zooplankton capture rate, expressed as the number of prey items captured per polyp and hour, was calculated using the following equation (Coma et al. 1994):
where C is the number of prey captured per polyp per hour, N is the number of prey items per polyp, t is time (in hours) and D is digestion time (in hours).
The assumptions of normality and homoscedasticity were tested using the Kolmogorov–Smirnov and the Levene’s tests, respectively. The homoscedasticity assumption was satisfied, but not the former. Differences in prey per polyp, prey size, prey capture rate and prey ingestion rate throughout the year were then analyzed using the Kruskal–Wallis test.
Biomass
Prey biomass was estimated from biovolumes (Sebens and Koehl 1984), using conversion factors for wet weight (1.025; Hall et al. 1970), dry weight (13% of wet weight, Beers 1966) and carbon content (45% of dry weight, Biswas and Biswas 1979). The biomass of L. sarmentosa colonies was estimated after rinsing to remove salts. Dry mass (DM) was determined by drying at 90°C for 24 h, and ash-free dry mass (AFDM), by combustion at 450°C for 5 h.
Feeding of L. sarmentosa colonies on nanoeukaryotes, diatoms, ciliates (i.e. live carbon <100 µm) and on detrital particulate organic carbon (POC) (Ribes et al. 2003) has been considered to estimate the effect of the species population on seston abundance.
Results
Zooplankton dynamics
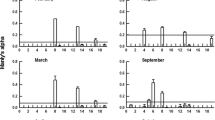
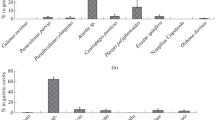
On the basis of abundance, zooplankton was classified in three main categories. Category 1 represented groups that were always present and contributed significantly to the total share, such as copepods (58% of the total), nauplii (20% of the total) and eggs (10%). These were the three dominant groups throughout the annual cycle and accounted for 80% (±8%) of the total plankton over the year. Category 2 included groups that were not always present, but were quantitatively significant at certain times of the year, such as bivalve larvae (6–19% from December to February), appendicularians (8–18% from June to August), protozoans (6–12% from October to January), polychaetes (6–9% from November to December) and tintinids (17% in August). Category 3 consisted of groups with small quantitative contributions. In all cases, the remaining groups accounted for about 5% or less of the total zooplankton catch (Table 1).
Total zooplankton abundance was highly variable within the study period, ranging between 369 and 4,015 ind. m−3, with an average of 1,624 ind. m−3 (±1,090 SD). The highest value was recorded in July, but several peaks occurred throughout the year (Table 1). Therefore, total zooplankton abundance showed high variability, and no clear seasonal pattern was evident.
Feeding on zooplankton
Most of the prey items observed inside the gastrovascular cavity of Leptogorgia sarmentosa were zooplankton (Table 2). Phytoplankton were not quantitatively studied here, since feeding on these organisms has been accurately examined with incubation chamber experiments (Ribes et al. 2003). The most abundant prey were eggs and invertebrate larvae, particularly bivalve larvae. These groups accounted for 66% of all items captured throughout the year; only on a few occasions were other groups observed (protozoans and copepods, Table 2). L. sarmentosa grazed on zooplankton prey that ranged in size from 80 to 340 μm, but items were particularly abundant between 80 and 200 μm (88%), and were characterized by low mobility. The mean percentage of polyps with food was 40.5% and ranged from 12% to 86% throughout the year. The mean number of prey items per polyp ranged from 0.22 to 1.74, although on a few occasions (11 of 1,250 polyps) polyps containing from 5 to 34 prey items were observed.
Although the guts of more than half the polyps were empty, ambient concentrations of all zooplankton groups combined did not contribute to explaining prey capture of the species, which has been described as percentage of polyps with food (full polyps) and as number of prey in 50 polyps (Table 3). Two observations indicate maximum efficiency of prey capture between 150 and 200 μm. Firstly, the prey size spectrum shows an almost normal distribution, with the largest share of prey between 150 and 200 μm (Fig. 1). Secondly, capture rates for specific prey correlated with ambient concentration for bivalve larvae, but not for the smallest and largest prey (Table 4). Bivalve larvae between 165 and 199 μm accounted for 60% of prey items between 150 and 200 μm.
The number of prey per polyp in the gastrovascular cavity of the L. sarmentosa polyps exhibited a marked pattern of seasonal variation (Kruskal–Wallis test, H=21.13, df=3, P=0.0001). During spring and winter, the number of prey was about twice that during summer and fall (Fig. 2).
Prey digestion time
Prey digestion time is a crucial element in estimating ingestion rates from gut contents and may be affected by temperature. Consequently, we conducted prey digestion experiments at three temperatures that covered the annual temperature range at the study site. Clearance of the gut contents showed an exponential decrease in the number of prey items per polyp with time (Fig. 3). Digestion time was calculated from the slope of the regression lines, and it exhibited a clear pattern of decrease with temperature increase (Fig. 4). Prey digestion time increased about 150% as temperature was lowered from 21°C to 13°C (the approximate temperature range at the study site). Complete clearance of the stomach took 41 h at 13°C, 23 h at 17°C and 16 h at 21°C (Fig. 4). However, the last 10% of prey items were always copepod carapaces and a few crustacean fragments; items that are difficult to digest and that are not representative of the bulk of prey. In our view, application of the digestion time for these difficult to digest prey items to all prey would result in a substantial overestimation of digestion time. Therefore, to calculate the zooplankton prey capture rate, we used a digestion time that we considered representative of the bulk of prey (i.e. that at which 90% of the prey has been digested, corresponding to the coefficient 0.10 in Fig. 4). This coefficient is the value of the term ebx from the exponential regression (Fig. 3), where b is the slope and x is the time (in hours). The coefficients 0.10, 0.05 and 0.00 provide the time at which the number of prey per polyp is 10%, 5% and 0%, respectively, from the initial number of prey per polyp (Fig. 4). Therefore, the time at which 90% of the prey had been digested (coefficient 0.10) was 15 h at 13°C, 9 h at 17°C and 6 h at 21°C (Fig. 4).
Leptogorgia sarmentosa. Pattern of decrease in prey digestion time (hours) with temperature increase. The coefficient is the value of the term ebx from the exponential regression, where b is the slope and x is the time (in hours). The coefficients 0.10, 0.05 and 0.00 provide the time at which the number of prey per polyp is 10%, 5% and 0%, respectively, from the initial number of prey per polyp
Prey capture rate
Zooplankton prey capture rate (i.e. the number of prey items captured per polyp and hour) exhibited a marked seasonal variation (Kruskal–Wallis test, H=9.58, df=3, P=0.022; Fig. 5a). The highest rates were observed in spring (0.158±0.019 prey polyp−1 h−1; mean±SE), while the other seasons showed similar capture rates (summer: 0.103±0.017, fall: 0.082±0.010 and winter: 0.104±0.018 prey polyp−1 h−1; mean±SE). Because of their size and abundance, invertebrate larvae were the main contributors to the bulk of zooplankton prey items in terms of biomass (53%, more than half of which were bivalve larvae). Ingestion rate in terms of biomass (μg C polyp−1 h−1) showed a similar trend (Fig. 5c), but the differences among the seasons were attenuated by seasonal differences in prey size (Kruskal–Wallis test, H=55.46, df=3, P=0.0001; Fig. 5b). Thus, the ingestion rate did not significantly vary over the year (Kruskal–Wallis test, H=6.04, df=3, P=0.1098; Fig. 5c). The average ingestion rate over the year was 0.019±0.002 μg C polyp−1 h−1 (mean±SE), corresponding to 136±14 μg C g−1 AFDM h−1. This value accounts for an average annual ingestion rate of 3,260±340 μg C g−1 AFDM day−1 (mean±SE).
Discussion
Zooplankton diet
The zooplankton diet of Leptogorgia sarmentosa was made up mainly of small (80–200 µm) and low-motile (i.e. eggs and larvae) prey items. Although a wide spectrum of other zooplankton was also found, including copepods up to 340 µm, they were not quantitatively relevant. Other passive suspension feeders also feed on non-motile zooplankton of similar size (Lewis 1982; Sebens and Koehl 1984; Coma et al. 1994; Fabricius et al. 1995; Ribes et al. 1998). This behavior appears to be related to the relatively limited variety and low density of nematocysts found in octocoral (Mariscal and Bigger 1977); consequently, most prey items captured were those with little capacity to escape.
Bivalve larvae were conspicuous prey items of L. sarmentosa and accounted for 21% of the total (Table 2). The positive correlation between the capture rate and ambient concentration of bivalve larvae indicates that feeding on these larvae may peak during adult spawning. Little is known about the role of predation in early stages of bivalve development (Ólaffson et al. 1994; Beukema et al. 2001). Further research should be conducted to determine whether predation by octocorals and other suspension feeders could be a detectable factor that affects bivalve recruitment.
Gut contents
Gut contents of L. sarmentosa exhibited an average of 0.68±0.86 prey polyp−1, a value similar to those previously estimated for temperate octocorals (Alcyonium siderium, Sebens and Koehl 1984; Paramuricea clavata, Coma et al. 1994), and one which differs markedly from the relatively low gut contents observed in tropical octocorals (usually below 0.3 prey polyp−1, Lewis 1982; Fabricius et al. 1995). This appears to be related to the fact that most temperate octocorals are exclusively heterotrophic (but see Capnella gaboensis, Farrant et al. 1987), while most tropical octocorals examined so far have symbiotic zooxanthellae (but see Dendronephthya hemprichi, Fabricius et al. 1998). Gut contents in temperate octocorals are associated with some of the highest values observed in reef hexacoral species, which are usually below 0.4 prey polyp−1 (Porter 1974; Johnson and Sebens 1993; Sebens et al. 1996). These values are also higher than those observed for zoanthid species, which are always below 0.12 prey polyp−1 (Sebens 1977).
Gut content examination showed that the effect of temperature on prey digestion time strongly modified the pattern of seasonal variation in prey per polyp and allowed us to estimate zooplankton prey capture and ingestion rate over the annual cycle.
Prey digestion time
Digestion time is a crucial factor in estimates of zooplankton prey capture rates, which have received relatively little attention for anthozoans (Sebens 1987). Digestion time exhibited a clear pattern of decrease with temperature increase, a pattern not previously documented in anthozoans. This result was expected because of the general tendency of chemical reaction rates to increase with temperature. The pattern observed was similar to those previously reported in hydrozoans (Kinne and Paffenhöfer 1965; Christensen 1967) and scyphomedusae (Purcell 1992). The largest temperature effect on digestion time is in the hydrozoan species Hydractinia echinata: digestion time was >40 h at 4°C, but only 5 h at 16°C (Christensen 1967). The estimated digestion times (6–15 h) were within the range of values reported for anthozoans (3–14 h, Boschma 1925; Yonge and Nicholls 1930; Porter 1974; Sebens and Koehl 1984; Barange and Gili 1988; Coma et al. 1994).
Prey capture rate
Flow speed and prey concentration are crucial factors that affect the efficiency with which particles are captured in organisms like octocorals (Shimeta and Jumars 1991). In our study sampling over the annual cycle was always conducted during the same time period to avoid any potential diel cycle effect. In our study area, zooplankton exhibited a marked diel cycle in open waters (Estrada et al. 1985; Siokou-Frangou 1996), which is characteristic of many temperate areas. However, few studies have addressed the near-substratum zooplankton at such fine spatial and temporal scales. Current knowledge about the dynamics of this zooplankton comes mainly from studies of benthic suspension feeders. In general, the concentrations of various zooplankton taxa change during the diel cycle, while others stay relatively constant. However, no clear diel pattern either in size or in abundance of zooplankton has been observed (Sebens and Koehl 1984; Coma et al. 1994; Gili et al. 1996). These studies suggest that in temperate areas near-substratum zooplankton does not exhibit any clear diel pattern of size or abundance, in contrast to that in coral reef systems, where a strong pattern of upward migration at dusk and downward migration at dawn is observed (e.g. Alldredge and King 1977; Ohlhorst 1982). Therefore, the time period during which sampling was conducted (9–11 h) did not introduce any significant bias on the estimate of the annual pattern of prey capture rates.
The results of gut content examinations integrate the effect of prey concentration and flow speed on the zooplankton prey capture rate of L. sarmentosa. Although there are no flow speed data available, it was surprising to observe the absence of a correlation between total ambient plankton concentration and capture rates for all but bivalve larvae. This result indicates the primary role of flow speed on prey capture rate (Dai and Lin 1993; Sebens et al. 1996; Fabricius et al. 1995). With regard to the small zooplankton taxa, the average widths of eggs (90 μm) and nauplii (112 μm) were below or to close to the size of the mesh (100 μm), and therefore our sampling probably underestimated the abundance of these taxa (UNESCO 1968). However, the results of the digestion experiments indicate that prey were probably captured over the previous 6–15 h, depending on the temperature. Therefore, the best estimate of prey availability to contrast with capture rates is not the zooplankton present at the time of collection, but the average of that present over several hours earlier. The higher prey capture and ingestion rates for L. sarmentosa in spring appear to be related to the higher values of flow speed in the area during that season (Font et al. 1995). However, in terms of ingestion rate, the higher prey capture rate observed in spring was attenuated by the differences in prey size among the seasons. Current knowledge about the dynamics of near-bottom zooplankton indicates high short-term variability in abundance, lacking any clear seasonal pattern (Coma et. al. 1994; Calbet et al. 2001). The variation in prey size observed may be related to the succession of taxa that the zooplankton community displays throughout the year (Calbet et al. 2001). Therefore, despite the marked seasonal pattern in gut contents, zooplankton ingestion rates did not significantly vary over the annual cycle.
Ingesta of the species
To estimate total ingesta of L. sarmentosa, we should also consider feeding on detrital POC and live carbon (data from Rossi 2002; Ribes et al. 2003; Table 5). The coenenchyme dry mass of the L. sarmentosa colonies was 56±2% (mean±SE) of total DM, and the AFDM of the colonies was 32±1% of the dry mass. These proportions allowed us to estimate that, on average, the colonies ingested 0.5% of their total body mass daily (all seston fractions included) or 0.8% of the coenenchyme mass. This is a very low ingestion rate compared to that of other anthozoan taxa (4.5–6%) and of hydrozoan taxa [10–60%, see Table 2 in Barange et al. (1989) for a review of the literature]. To examine whether our ingesta estimates cover the metabolic expenses of the species, we used respiration rate data from a previous study. Ribes et al. (2003) estimated that the respiration rate of the species in spring (temperature ranging between 16.5°C and 18°C) was 0.55 mg O2 g−1 AFDM h−1, which is equivalent to 155 μg C g−1 AFDM h−1. This value would represent a maximum of 3,720 μg C g−1 AFDM day−1 if we consider that the polyps are expanded the whole day. Average zooplankton ingesta (3,260 μg C g−1 AFDM day−1) could barely account for the metabolic cost of respiration. However, several factors should be considered. First, L. sarmentosa does not feed on zooplankton alone, but also on detrital POC and live carbon <100 μm. If all seston sources are considered, the species ingested an average of 6,600 μg C g−1 AFDM day−1. Second, the respiration rate was estimated for expanded polyps. Therefore, the respiration rate is probably lower than the values estimated, because, on average, polyps are expanded only part of the time (Coma et al. 1994, and unpublished data) and the contraction of polyps reduces the rate of respiration between 10% and 60% (Sebens 1987; Fabricius and Klumpp 1995; Coma et al. 2002). Third, respiration was estimated in spring, one of the periods of higher investment of the species in secondary production (Rossi 2002), and, therefore, the respiration rate estimated includes the cost associated with the synthesis of new tissue (Coma et al. 2002). Fourth, the estimated values of total ingesta should be considered as conservative, because feeding on detrital POC and live carbon <100 μm (Ribes et al. 2003) was studied at a low flow speed (1.2 cm s−1) and capture rates increase several-fold with an increase in flow speed (Fabricius et al. 1995). Thus, although this estimate may be an underestimate of the ingesta of L. sarmentosa, it can largely account for the respiration rate of the species.
Effect of octocoral predation on seston abundance
The density of L. sarmentosa in the study area was 1.5 colonies m−2, with a mean height of 23 cm (Rossi 2002), which represents a standing stock of 1.2 g AFDM m−2. To estimate the effect of the octocoral population on seston abundance, we also considered feeding of the species on detrital POC and live carbon <100 μm (data from Rossi 2002; Ribes et al. 2003; Table 5). At the estimated ingestion rates (1,928–14,006 μg C g−1 AFDM day−1) on all seston fractions (i.e. zooplankton, detrital POC and live carbon <100 μm), the population of L. sarmentosa removed between 2.3 and 16.8 mg C m−2 day−1 from the adjacent water column. Higher population densities have been reported in other locations such as Banyuls-sur-Mer, France (2.4 colonies m−2, Rossi 2002), and La Spezia, Italy (6.8 colonies m−2, corresponding to a standing stock of 5.44 g−1 AFDM m−2, Mistri 1995). The ingestion rate of L. sarmentosa on all seston fractions (0.007±0.003 g C g−1 AFDM day−1; mean±SD) is among the values reported for octocorals (Plexaura flexuosa: 0.045×10−3 g C g−1 AFDM day−1, Ribes et al. 1998; Dendronephthya hemprichi: 0.008 g C g−1 AFDM day−1, Fabricius et al. 1998; Paramuricea clavata: 0.002 g C g−1 AFDM day−1, Coma et al. 1994; Ribes et al. 1999; octocoral community: ~0.010 g C g−1 AFDM day−1, Fabricius and Dommisse 2000). Because of the relatively low density of L. sarmentosa, the impact of this population on the water column (2.3–16.8 mg C m−2 day−1) is among the lowest values reported for octocorals (Plexaura flexuosa: 0.15 mg C m−2 day−1, Ribes et al. 1998; Dendronephthya hemprichi: 451 mg C m−2 day−1, Fabricius et al. 1998; Paramuricea clavata: up to 170 mg C m−2 day−1, Coma et al. 1994; Ribes et al. 1999; octocoral community: ~2.5 g C m−2 day−1, Fabricius and Dommisse 2000), but within the range reported for other passive suspension feeders, including most hydrozoans (between 6 and 66 mg C m−2 day−1, Gili et al. 1998). These observations indicate that predation by macroinvertebrates on plankton populations and suspended detrital POC should be considered in energy transfer processes in littoral areas, since even species with a low abundance may have a detectable impact.
References
Alldredge AL, King JM (1977) Distribution and substrate preferences of demersal reef zooplankton at Lizard Island Lagoon, Great Barrier Reef. Mar Biol 41:317–333
Barange M, Gili JM (1988) Feeding cycles and prey capture in Eudendrium racemosum (Cavolini, 1785). J Exp Mar Biol Ecol 115:281–293
Barange M, Zabala M, Riera T, Gili JM (1989) A general approach to the in situ energy budget of Eudendrium racemosum (Cnidaria, Hydrozoa) in the western Mediterranean. Sci Mar 53:423–427
Beers JR (1966) Studies on the chemical composition of the major zooplankton groups in the Sargasso Sea off Bermuda. Limnol Oceanogr 11:520–528
Beukema JJ, Dekker R, Essink K, Michaelis H (2001) Synchronized reproductive success of the main bivalve species in the Wadden Sea: causes and consequences. Mar Ecol Prog Ser 211:143–155
Bingham BL, Walters LJ (1989) Solitary ascidians as predators of invertebrate larvae: evidence from gut analyses and plankton samples. J Exp Mar Biol Ecol 131:147–159
Biswas AK, Biswas MR (1979) Handbook of environmental data and ecological parameters, vol 6. Environmental sciences and applications. Pergamon, Oxford
Boschma H (1925) On the feeding reactions and digestion in the coral polyp Astrangia danae, with notes on its symbiosis with zooxanthellae. Biol Bull (Woods Hole) 49:407–439
Calbet A, Garrido S, Saiz E, Alcaraz M, Duarte CM (2001) Annual zooplankton succession in coastal NW Mediterranean waters: the importance of the smaller size fractions. J Plankton Res 23:319–331
Christensen HE (1967) Ecology of Hydractinia echinata. I. Feeding biology. Ophelia 4:245–275
Clarke A (1988) Seasonality in the Antarctic marine environment. Comp Biochem Physiol B 90:461–473
Cloern JE (1982) Does the benthos control phytoplankton biomass in south San Francisco Bay? Mar Ecol Prog Ser 9:191–202
Coffroth MA (1984) Ingestion and incorporation of coral mucus aggregates by a gorgonian soft coral. Mar Ecol Prog Ser 17:193–199
Coma R, Ribes M (2003) Seasonal energetic constraints in Mediterranean benthic suspension feeders: effects at different levels of ecological organization. Oikos 101:205–215
Coma R, Gili JM, Zabala M, Riera T (1994) Feeding and prey capture cycles in the aposymbiontic gorgonian Paramuricea clavata. Mar Ecol Prog Ser 115:257–270
Coma R, Gili JM, Zabala M (1995) Trophic ecology of a benthic marine hydroid, Campanularia everta. Mar Ecol Prog Ser 119:211–220
Coma R, Ribes M, Orejas C, Gili JM (1999) Prey capture by a benthic coral reef hydrozoan. Coral Reefs 18:141–145
Coma R, Ribes M, Gili JM, Zabala M (2000) Seasonality in coastal benthic ecosystems. Trends Ecol Evol 15:448–453
Coma R, Ribes M, Gili JM, Zabala M (2002) Seasonality of in situ respiration rate in three temperate benthic suspension feeders. Limnol Oceanogr 47:324–331
Dai CF, Lin MC (1993) The effects of flow on feeding of three gorgonians from southern Taiwan. J Exp Mar Biol Ecol 173:57–69
Davenport J, Smith RJJ, Packer M (2000) Mussels Mytilus edulis: significant consumers and destroyers of mesozooplankton. Mar Ecol Prog Ser 198:131–137
Estrada M, Vives F, Alcaraz M (1985) Life and productivity of the open sea. In: Margalef R (ed) Western Mediterranean. Pergamon, Oxford, pp 148–197
Fabricius KE, Dommisse M (2000) Depletion of suspended particulate matter over coastal reef communities dominated by zooxanthellate soft corals. Mar Ecol Prog Ser 196:157–167
Fabricius KE, Klumpp DW (1995) Widespread mixotrophy in reef-inhabiting soft corals: the influence of depth, and colony expansion and contraction on photosynthesis. Mar Ecol Prog Ser 125:195–204
Fabricius KE, Genin A, Benayahu Y (1995) Flow-dependent herbivory and growth in zooxanthellae-free soft corals. Limnol Oceanogr 40:1290–1301 (Erratum Limnol Oceanogr 41:1520)
Fabricius KE, Yahel G, Genin A (1998) In situ depletion of phytoplankton by an azooxanthellate soft coral. Limnol Oceanogr 43:354–356
Farrant PA, Borowitzka MA, Hinde R, King RJ (1987) Nutrition of the temperate Australian soft coral Capnella gaboensis. II. The role of zooxanthellae and feeding. Mar Biol 95:575–581
Font J, Garcia E, Gorriz EG (1995) The seasonality of mesoscale motion in the northern current of the western Mediterranean: several years of evidence. Oceanol Acta 18:207–219
Fréchette M, Butman CA, Geyer WR (1989) The importance of boundary-layer flow in supplying phytoplankton to the benthic suspension feeder, Mytilus edulis L. Limnol Oceanogr 34:19–36
Gili JM, Coma R (1998) Benthic suspension feeders: their paramount role in littoral marine food webs. Trends Ecol Evol 13:316–321
Gili JM, Hughes RG, Alvà V (1996) A quantitative study of feeding by the hydroid Tubularia larynx. Sci Mar 60:43–54
Gili JM, Alvà V, Coma R, Orejas C, Pagès F, Ribes M, Zabala M, Arnts W, Bouillon J, Boero F, Hughes RG (1998) The impact of small benthic suspension feeders in shallow marine ecosystems: the hydroids as an example. Zool Verh (Leiden) 323:99–105
Graf G, Schulz R, Peinert R, Meyer-Reil LA (1983) Benthic response to sedimentation events during autumn to spring at a shallow-water station in the western Kiel Bight. I. Analysis of processes on a community level. Mar Biol 77:235–246
Hall DJ, Cooper WE, Werner EE (1970) An experimental approach to the production dynamics and structure of freshwater animal communities. Limnol Oceanogr 15:838–928
Johnson AS, Sebens KP (1993) Consequences of a flattened morphology: effects of flow on feeding rates of the scleractinian coral meandrina meandrites. Mar Ecol Prog Ser 99:99–114
Kim K, Lasker HR (1997) Flow-mediated resource competition in suspension-feeding gorgonian Plexaura homomalla (Esper.). J Exp Mar Biol Ecol 215:49–64
Kimmerer WJ, Gartside E, Orsi JJ (1994) Predation by an introduced clam as the likely cause of substantial declines in zooplankton of San Francisco Bay. Mar Ecol Prog Ser 113:81–93
Kinne O, Paffenhöfer GA (1965) Hydranth structure and digestion rate as a function of temperature and salinity in Clava multicornis (Cnidaria, Hydrozoa). Helgol Wiss Meeresunters 13:329–341
Kinzie RA III (1973) The zonation of West Indian gorgonians. Bull Mar Sci 23:93–155
Lasker HR (1981) A comparison of the particulate feeding abilities of three species of gorgonian soft coral. Mar Ecol Prog Ser 5:61–67
Lewis JB (1982) Feeding behaviour and feeding ecology of the Octocorallia (Coelenterata: Anthozoa). J Zool (Lond) 196:371–384
Mariscal RN, Bigger CH (1977) Possible ecological significance of octocoral epithelial ultrastructure. In: Taylor DL (ed) Proc 3rd Int Coral Reef Symp, vol 1. Miami School of Marine and Atmospheric Sciences, University of Miami, Miami, pp 127–134
Mistri M (1995) Population structure and secondary production of the Mediterranean octocoral Leptogorgia sarmentosa (L., 1758). Mar Ecol 16:181–188
Mitchell ND, Dardeau MR, Schroeder WW, Benke AC (1992) Secondary production of gorgonian corals in the northern Gulf of Mexico. Mar Ecol Prog Ser 87:275–281
Officer CB, Smayda TJ, Mann R (1982) Benthic filter feeding: a natural eutrophication control. Mar Ecol Prog Ser 9:203–210
Ohlhorst SL (1982) Diel migration of demersal reef zooplankton. J Exp Mar Biol Ecol 41:317–333
Ólafsson EB, Peterson CH, Ambrose WG Jr (1994) Does recruitment limitation structure populations and communities of macro-invertebrates in soft marine sediments: the relative significance of pre- and post-settlement processes. Oceanogr Mar Biol Annu Rev 32:65–109
Paffenhöfer GA (1968) Nahrungsaufnahme, Stoffumsatz und Energiehaushalt des marinen Hydroidpolypen Clava multicornis. Helgol Wiss Meeresunters 8:1–44
Pêrès JM (1967) The Mediterranean benthos. Oceanogr Mar Biol Annu Rev 5:449–533
Porter JW (1974) Zooplankton feeding by the Caribbean reef-building coral Monastrea cavernosa. In: Cameron AM, et al (eds) Proc 2nd Int Coral Reef Symp, vol 1. Great Barrier Reef Committee, Brisbane, pp 111–125
Purcell JE (1992) Effects of predation by the scyphomedusan Chrysaora quinquecirrha on zooplankton populations in Chesapeake Bay, USA. Mar Ecol Prog Ser 87:65–76
Ribes M, Coma R, Gili JM (1998) Heterotrophic feeding by gorgonian corals with symbiotic zooxanthellae. Limnol Oceanogr 43:1170–1179
Ribes M, Coma R, Gili JM (1999) Heterogeneous feeding in benthic suspension feeders: the natural diet and grazing rate of the temperate gorgonian Paramuricea clavata (Cnidaria: Octocorallia) over a year cycle. Mar Ecol Prog Ser 183:125–137
Ribes M, Coma R, Rossi S (2003) Natural feeding of the temperate asymbiotic octocoral gorgonian Leptogorgia sarmentosa (Cnidaria: Octocorallia). Mar Ecol Prog Ser 254:141–150
Riisgård HU, Jensen AS, Jürgensen C (1998) Hydrography, near-bottom currents, and grazing impact of the filter-feeding ascidian Ciona intestinalis in a Danish fjord. Ophelia 49:1–16
Rossi S (2002) Environmental factors affecting the trophic ecology of benthic suspension feeders. PhD thesis, University of Barcelona, Barcelona
Sebens KP (1977) Autotrophic and heterotrophic nutrition of coral reef zoanthids. In: Taylor DL (ed) Proc 3rd Int Coral Reef Symp, vol 1. Miami School of Marine and Atmospheric Sciences, University of Miami, Miami, pp 397–406
Sebens KP (1987) Coelenterata. In: Pandian TJ, Vernberg FJ (eds) Animals energetics, vol 1. Academic, San Diego, pp 55–120
Sebens KP, Koehl MAR (1984) Predation on zooplankton by the benthic anthozoans Alcyonium sidereum (Alcyonacea) and Metridium senile (Actiniaria) in the New England subtidal. Mar Biol 81:255–271
Sebens KP, Vandersall KS, Savina LA, Graham KR (1996) Zooplankton capture by two scleractinian corals, Madracis mirabilis and Monastrea cavernosa, in a field enclosure. Mar Biol 127:303–317
Shimeta J, Jumars PA (1991) Physical mechanisms and rates of particle capture by suspension feeders. Oceanogr Mar Biol Annu Rev 29:191–257
Siokou-Frangou I (1996) Zooplankton annual cycle in a Mediterranean coastal area. J Plankton Res 18:203–223
Sokal R, Rohlf FJ (1995) Biometry. The principles and practice of statistics in biological research, 3rd edn. Freeman, New York
Starmans A, Gutt J, Arntz WE (1999) Mega-epibenthic communities in Arctic and Antarctic shelf areas. Mar Biol 135:269–280
True MA (1970) Étude quantitative de quattre peuplements sciaphiles sur substrat rocheux dans la région marseilles. Bull Inst Oceanogr Monaco 1410:1–48
UNESCO (United Nations Educational, Scientific and Cultural Organization) (1968) Zooplankton sampling. Monographs on oceanographic methodology. UNESCO, Paris
Weinberg S (1978) Mediterranean octocorallian communities and the abiotic environment. Mar Biol 49:41–57
Wendt PH, van Dolah RF, O’Rourke CB (1985) A comparative study of the invertebrate macrofauna associated with seven sponge and coral species collected from the South Atlantic Bight. J Elisha Mitchell Sci Soc 101:187–203
Yonge CM, Nicholls AG (1930) Studies on the physiology of corals. II. Digestive enzymes with notes on the speed of digestion. Sci Rep Great Barrier Reef Exped 1:15–57
Acknowledgements
The authors thank I. Llobet for examining the zooplankton samples and M. Cascante, A. Cugat and A. Liebt for examining the gut contents. The experiments comply with the current laws of Spain, where they were conducted. Support for this work was provided by a F.P.I. fellowship from the Spanish Ministry of Education and Culture (MEC), which was granted to S.R. under D.G.I.C.Y.T. project PB94-0014-C02-01, a “Ramón y Cajal” research contract granted to M.R. by the MEC, a research contract granted to R.C. by the MEC, and by D.G.I.C.Y.T. projects REN2000-0633-C03-01/MAR and REN2002-01631/MAR.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S.A. Poulet, Roscoff
Rights and permissions
About this article
Cite this article
Rossi, S., Ribes, M., Coma, R. et al. Temporal variability in zooplankton prey capture rate of the passive suspension feeder Leptogorgia sarmentosa (Cnidaria: Octocorallia), a case study. Marine Biology 144, 89–99 (2004). https://doi.org/10.1007/s00227-003-1168-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1168-7