Abstract
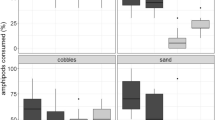
Intertidal endobenthic bivalves are often dislodged from sediments by hydrodynamic forces. As a result, they encounter the dangers of predation and desiccation, which are generally harsh near the sediment surface. To cope with such dangers, the bivalves possibly possess: (1) a strong body to endure predation and desiccation stress, (2) quick mobility to avoid the stresses, or (3) a high growth rate for attaining a size refuge. The present study examined which of these modes are adopted by the subtropical cobbled-shore Venus clams Gafrarium tumidum (Röding, 1798) and Ruditapes variegatus (Sowerby, 1852), revealing the following interspecific differences. (1) G. tumidum survived better than R. variegatus did in harsh experimental conditions, namely: the experimental cages exposed to predation and desiccation on a cobbled shore; a laboratory aquarium with a predatory crab Scylla serrata; and ovens with high temperatures (27°C and 34°C). (2) R. variegatus was more mobile than G. tumidum was, digging into the sediment on a cobbled shore more rapidly at both high and low tides. (3) The two species with shell lengths 20–30 mm showed similar growth rates (median: −0.2 to 44.5 μm day−1) in seasonal mark–recapture surveys over 2 years. Overall, to cope with the dangers of predation and desiccation G. tumidum appears to have a strong body, while R. variegatus displays rapid mobility, and neither species seems to attain a size refuge through rapid growth. Such species-specific modes are discussed in relation to the interspecific differences found in shell morphology.


Similar content being viewed by others
References
Arnold WS (1984) The effects of prey size, predator size and sediment composition on the rate of predation of the blue crab, Callinectes sapidus Rathbun, on the hard clam, Mercenaria mercenaria (Linné). J Exp Mar Biol Ecol 80:207–219
Baron J (1992) Reproductive cycles of the bivalve molluscs Atactodea striata (Gmelin), Gafrarium tumidum Röding and Anadara scapha (L.) in New Caledonia. Aust J Mar Freshw Res 43:393–402
Berg CJ, Alatalo P (1985) Biology of the tropical bivalve Asaphis deflorata (Linné, 1758). Bull Mar Sci 37:827–838
Bertness MD, Garrity SD, Levings SC (1981) Predation pressure and gastropod foraging: a tropical–temperate comparison. Evolution 35:995–1007
Blundon JA, Kennedy VS (1982a) Mechanical and behavioral aspects of blue crab, Callinectes sapidus (Rathbun), predation on Chesapeake Bay bivalves. J Exp Mar Biol Ecol 65:47–65
Blundon JA, Kennedy VS (1982b) Refuges for infaunal bivalves from blue crab, Callinectes sapidus (Rathbun), predation in Chesapeake Bay. J Exp Mar Biol Ecol 65:67–81
Boulding EG (1984) Crab-resistant features of shells of burrowing bivalves: decreasing vulnerability by increasing handling time. J Exp Mar Biol Ecol 76:201–223
Brown JH, Lomolino MV (1998) Biogeography. Sinauer, Sunderland, Mass.
Charnov EL (1976) Optimal foraging: attack strategy of a mantid. Am Nat 110:141–151
Commito JA (1982) Effects of Lunatia heros predation on the population dynamics of Mya arenaria and Macoma balthica in Maine, USA. Mar Biol 69:187–193
Commito JA, Ambrose WG (1985) Multiple trophic levels in soft-bottom communities. Mar Ecol Prog Ser 26:283–289
Commito JA, Currier CA, Kane LR, Reinsel KA, Ulm IM (1995) Dispersal dynamics of the bivalve Gemma gemma in a patchy environment. Ecol Monogr 65:1–20
Eggleston DB (1990) Functional responses of blue crabs Callinectes sapidus Rathbun feeding on juvenile oysters Crassostrea virginica (Gmelin): effects of predator sex and size, and prey size. J Exp Mar Biol Ecol 143:73–90
Eggleston DB, Lipcius RN, Hines AH (1992) Density-dependent predation by blue crabs upon infaunal clam species with contrasting distribution and abundance patterns. Mar Ecol Prog Ser 85:55–68
Emerson CW, Grant J (1991) The control of soft-shell clam (Mya arenaria) recruitment on intertidal sandflats by bedload sediment transport. Limnol Oceanogr 36:1288–1300
Grant J (1981) Sediment transport and disturbance on an intertidal sandflat: infaunal distribution and recolonization. Mar Ecol Prog Ser 6:249–255
Haddon M, Wear RG, Packer HA (1987) Depth and density of burial by the bivalve Paphies ventricosa as refuges from predation by the crab Ovalipes catharus. Mar Biol 94:25–30
Hilgerloh G (1997) Predation by birds on blue mussel Mytilus edulis beds of the tidal flats of Spiekeroog (southern North Sea). Mar Ecol Prog Ser 146:61–72
Hines AH, Whitlatch RB, Thrush SF, Hewitt JE, Cummings VJ, Dayton PK, Legendre P (1997) Nonlinear foraging response of a large marine predator to benthic prey: eagle ray pits and bivalves in a New Zealand sandflat. J Exp Mar Biol Ecol 216:191–210
Hughes RN, Elner RW (1979) Tactics of a predator, Carcinus maenas, and morphological responses of the prey, Nucella lapillus. J Anim Ecol 48:65–78
Jangoux M (1982) Food and feeding mechanisms: Asteroidea. In: Jangoux M, Lawrence JM (eds) Echinoderm nutrition. Balkema, Rotterdam, pp 117–159
Kurihara T (2002) Spatial and temporal fluctuation in the density of the intertidal limpet, Patelloida striata Quoy & Gaimard, on subtropical cobbled shores. J Moll Stud 68:83–90
Kurihara T, Takada Y, Kosuge T, Kobayashi M, Katoh M, Mito K (2000) Species composition of epifauna and infauna on intertidal boulder shores at Ishigaki Island in subtropical Japan. Bull Seikai Natl Fish Res Inst 78:31–47
Leonard GH, Bertness MD, Yund PO (1999) Crab predation, waterborne cues, and inducible defenses in the blue mussel, Mytilus edulis. Ecology 80:1–14
Matthiessen GC (1960) Intertidal zonation in populations of Mya arenaria. Limnol Oceanogr 5:381–388
Ota N, Tokeshi M (2000) Population analysis of Ruditapes variegatus (Sowerby) (Bivalvia: Veneridae) on an intertidal boulder shore. Venus Jpn J Malacol 59:29–36
Paine RT (1976) Size-limited predation: an observational and experimental approach with the Mytilus–Pisaster interaction. Ecology 57:858–873
Porter WP, Gates DM (1969) Thermodynamic equilibria of animals with environment. Ecol Monogr 39:227–244
Ricciardi A, Serrouya R, Whoriskey FG (1995) Aerial exposure tolerance of zebra and quagga mussels (Bivalvia: Dreissenidae): implications for overload dispersal. Can J Fish Aquat Sci 52:470–477
Robert R, Parra R (1991) Experimental study of predation by the gilthead bream Sparus aurata and the gray triggerfish Balistes capriscus on the Manila clam Ruditapes philippinarum (in French with English abstract). Aquat Living Resour 4:181–189
Rodrigues CL (1986) Predation of the naticid gastropod Neverita didyma (Röding) on the bivalve Ruditapes philippinarum (Adams & Reeve): evidence for a preference linked functional response. Publ Amakusa Mar Biol Lab Kyushu Univ 8:125–141
Saito H, Imabayashi H, Kawai K (1999) Growth of the bivalve-feeder Halla okudai (Polychaeta: Lysaretidae) under wild and rearing conditions, in relation to species and abundance of prey organisms. Fish Sci (Tokyo) 65:230–234
Sakurai I, Seto M (1999) Behavioral characteristics of the juvenile Japanese littleneck clam Ruditapes philippinarum in response to sand erosion and deposition (in Japanese with English abstract). Sci Rep Hokkaido Fish Exp Stn 54:41–46
Sakurai I, Seto M, Nakao S (1996) Effects of water temperature, salinity and substrata on burrowing behaviors of the three bivalves, Pseudocardium sachalinensis, Mactra chinensis, and Ruditapes philippinarum (in Japanese with English abstract). Nippon Suisan Gakkaishi 62:878–885
Savidge WB, Taghon GL (1988) The influence of passive advection on the colonization of two types of disturbance on an intertidal sandflat. J Exp Mar Biol Ecol 115:137–155
Seed R, Brown RA (1978) Growth as a strategy for survival in two marine bivalves, Cerastoderma edule and Modiolus modiolus. J Anim Ecol 47:283–292
Seitz RD, Lipcius RN, Hines AH, Eggleston DB (2001) Density-dependent predation, habitat variation, and the persistence of marine bivalve prey. Ecology 82:2435–2451
Sponaugle S, Lawton P (1990) Portunid crab predation on juvenile hard clams: effects of substrate type and prey density. Mar Ecol Prog Ser 67:43–53
Turner SJ, Grant J, Pridmore RD, Hewitt JE, Wilkinson MR, Hume TM, Morrisey DJ (1997) Bedload and water-column transport and colonization processes by post-settlement benthic macrofauna: does infaunal density matter? J Exp Mar Biol Ecol 216:51–75
Underwood AJ (1997) Experiments in ecology. Cambridge University Press, Cambridge, UK
Vermeij GJ (1973) Morphological patterns in high-intertidal gastropods: adaptive strategies and their limitations. Mar Biol 20:319–346
Vermeij GJ (1978) Biogeography and adaptation. Harvard University Press, Cambridge, Mass.
Vermeij GJ (1987) Evolution and escalation: an ecological history of life. Princeton University Press, Princeton, N.J.
Woodin SA (1978) Refuges, disturbance, and community structure: a marine soft-bottom example. Ecology 59:274–284
Zaklan SD, Ydenberg R (1997) The body size-burial depth relationship in the infaunal clam Mya arenaria. J Exp Mar Biol Ecol 215:1–17
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Engelwood Cliffs, N.J.
Zipser E, Vermeij GJ (1978) Crushing behavior of tropical and temperate crabs. J Exp Mar Biol Ecol 31:155–172
Zwarts L, Wanink J (1989) Siphon size and burying depth in deposit- and suspension-feeding benthic bivalves. Mar Biol 100:227–240
Acknowledgements
I am obliged to Drs. Y. Shimadzu, Y. Takada, S. Wada and an anonymous referee for their constructive comments regarding this manuscript. I thank Mrs. Y. Hosokawa for her help with the measurement of samples. I am indebted to the staff of the Ishigaki Tropical Station for their advice and assistance during the study. The present study was supported in part by the Fisheries Research Center Seeds Research Program. The experiments in the present study comply with the current laws of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Ikeda, Hakodate
Rights and permissions
About this article
Cite this article
Kurihara, T. Adaptations of subtropical Venus clams to predation and desiccation: endurance of Gafrarium tumidum and avoidance of Ruditapes variegatus . Marine Biology 143, 1117–1125 (2003). https://doi.org/10.1007/s00227-003-1158-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1158-9