Abstract
Content and distribution of basic proteins, histones, acidic proteins and DNA, as well as the interaction of basic proteins with DNA, were studied microfluorometrically within nucleoid bodies of the gigantic eubacterium Epulopiscium fishelsoni living in the guts of the algivorous surgeonfish Acanthurus nigrofuscus. The fine structure of the bacterial nucleoid was studied using transmission electron microscopy (TEM). The mean content of basic proteins, histones and acidic proteins per nucleoid was directly proportional both to cell volume and DNA amount, proving that these proteins are integral components of bacterial chromatin. The maximal DNA quantity of ~1012 base pairs nucleoid−1 was found in the largest specimens of 354,000 µm3 volume (150-fold more then in human lymphocyte). Binding of proteins to DNA was strongest in cup-like nucleoids at the end of the bacterial life cycle, and weakest in enlarged and elongated nucleoids in mid-cycle. Contact fluorescent microscopy and TEM revealed a non-homogenous distribution of these proteins within the nucleoids, as well as the presence of giant polytene chromosome(s). We assume that the unusual genetic and morphological peculiarities, particularly increased polyploidy and polyteny, as revealed in E. fishelsoni, are the results of specific adaptations to the chemical conditions in the host's gut.
Similar content being viewed by others
Introduction
Recent studies using modern methods, particularly fluorescent microscopy, have demonstrated numerous examples of complex organization of the prokaryotic genome, its variable size among species, and complex chromosome packaging and gene regulation (Sharpe and Errington 1999; Sharpe et al. 1999; Azam et al. 2000; Hiraga 2000; Holmes and Cozzarelli 2000; Jensen and Shapiro 2000; Lewis 2001). Where chromosome number (ploidy), linearity, packaging and gene regulation are concerned, numerous similar mechanisms are found in both prokaryotes and eukaryotes (Bendich and Drlica 2000).
The largest known eubacterium, Epulopiscium fishelsoni Montgomery and Pollak, 1988, a viviparous gut symbiont of the brown surgeonfish, Acanthurus nigrofuscus, first described in 1985 (Fishelson et al. 1985), has a maximal length of 500–600 µm and the greatest amount of DNA (Angert et al. 1993, 1996; Bresler et al. 1998). Angert et al. (1993), analyzing a small subunit of rRNA genes of E. fishelsoni, revealed its possible relation to Clostridium. The life cycle of E. fishelsoni in the fish gut (Bresler et al. 1998) comprises at least four stages: (1) polar cup-like nuceoid bodies (NB), with condensed functionally inactive DNA; (2) DNA decondensation and activation within growing NB and formation of a peripheral circular DNA layer; (3) formation of daughter cells within maternal bacteria; and (4) DNA condensation near poles of daughter cells and its pulling by the cap-like structures.
It is recognized that cells employ structural proteins to confine and structure their chromosomes (Sandman et al. 1998). To accomplish this prokaryotes have evolved a variety of small and basic, DNA-binding proteins, that include histones in Euryarcheota and histone-like nucleoid-structuring proteins (H-NS) in bacteria.
The object of this study was to identify the quantity and distribution of basic, histone-like and acidic proteins within the nucleoids, and their interaction with DNA. For these studies we used fluorescent cytochemistry, fluorescent microscopy, microfluorometry and transmission electron microscopy (TEM).
Materials and methods
Samples of Epulopiscium fishelsoni Montgomery and Pollak, 1988 were collected in the Gulf of Aqaba (Red Sea) from the small intestine of the surgeonfish Acanthurus nigrofuscus and fixed with 3.5% glutaraldehyde, formaldehyde or absolute methanol. Following aldehyde fixations for 24 h the samples were washed in a buffer solution of 0.88 M sucrose with 1% acacia and stored for 5–12 weeks at 2–4°C. Such storage protects the fine structure of the cells and even their enzymatic activity (Geyer 1973). Similar to our previous study (Bresler et al. 1998), in this study too the bacteria were measured using an ocular micrometer and following measurement divided into six size groups, from group I (smallest) to VI (largest). The fine structure of the NB was studied by TEM as well as contact microscopy. This last technique is a special form of water immersion microscopy with a high numerical aperture and an ultrathin water layer between the frontal lens and the object [for more about contact fluorescent microscopy see Bresler et al. (1998)]. Basic and acidic proteins were visualized with the fluorochrome brilliant sulfaflavine (BSF) at pH 8.0 and 2.8, respectively, with or without DNA extraction (Ruch 1973; Curtis and Cowden 1985). DNA was extracted with hot (95°C) 5% trichloroacetic acid for 15 min. Histones were visualized by the fluorescent compound dimethylaminonaphthalene sulfonic acid (DANSYL), also with and without DNA extraction (Clark 1987). DNA was visualized by ethidium bromide (Ruch 1973). Human lymphocytes were used as reference standard, and as revealed they have 6.0×109 base pairs. The quantity of proteins and DNA in nucleoid bodies was determined microfluorimetrically according to the technique described by Bresler et al. (1998). The results were processed by Student's test, and are given in arbitrary units (a.u.) as used in fluorescence microscopy, showing millivolts of photocurrent. The criterion of significance of the observed differences between the means (±95% confidence limits) was P<0.05.
Results
Microfluorimetric data show that the DNA content per NB in the largest (>500 µm) specimens of Epulopiscium fishelsoni is approximately 150-fold that of DNA in the common reference standard, the human lymphocyte. This, being proportional to the cell size, is equivalent to 6.0×109 bp×150=1012 bp in the largest binucleoid bacteria of 354,000 µm3 in volume. In contrast, the smallest specimens observed of an O-group and 169 µm3 contained ~3.3×108 base pairs. This appears to indicate that the maximal level of polyploidy in E. fishelsoni varies by approximately 3,000-fold, and is apparently the highest known for bacteria, and the largest DNA content per cell known among the studied eukaryotic and prokaryotic cells (Alberts et al. 1994; Bendich and Drlica 2000).
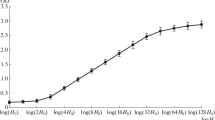
Microfluorometrical determination of basic proteins, histones and acidic proteins revealed that their mean content per NB in the binucleoid E. fishelsoni correlated significantly to the cell volume (R=0.96, P<0.05) and content of DNA (Fig. 1). Uninucleoid bacteria contained a double quantity of these proteins as compared to single NB of binucleoid bacteria from the same size group. There was a significant correlation between basic proteins and histones. These data show that the DNA/protein ratios in NB are constant. Fluorescence intensities of fluorochrome-treated basic proteins and histones within NB changed following DNA extraction (Table 1). According to our data, the strongest DNA–protein interactions are found in cup-like, resting NB, and the weakest in elongated and enlarging NB (P<0.05). For example, in the cup-like condensed nucleoids of the smallest bacteria, group I, the fluorescence with DNA was 164.7 a.u. and in those without DNA 104.7; in the largest bacteria, group VI, the values were 5281.9 and 3343.4 a.u., respectively. Calculations show (Table 1) that at the cup-like stage, the differences of fluorescence in the various groups of bacteria were approximately 36%, in the early decondensed stage 27%, and in the advanced decondensation of nucleoids 19.5%. These data provide evidence that DNA–protein interactions within NB protect the reactive groups of protein from fluorochrome molecules. Contact fluorescence microscopy of NB sections revealed a non-homogenous distribution of basic proteins, histones and DNA, brightly fluorescent, elongated and banded, cable-like structures, containing both proteins and DNA, visible on a low-fluorescent background. In contrast, acidic proteins formed separate areas, two of which were found near poles of the NB. The above-mentioned cable-like bands within the NB of E. fishelsoni, which were also exposed by TEM, are continuous structures that often formed helices with coil diameters of 1.3–1.5 to 2.6–3.0 μm (Fig. 2A). These cable-like and helical chromosomes roll up to form larger clews in the NB (Fig. 2B), along which transverse pale and dark bands of chromomeres are visible, providing a clear picture of polyteny (Fig. 2C). TEM also exposed areas of low electron densities that looked like "empty areas" in the NB (Fig. 2B).
Epulopiscium fishelsoni. Mean content of basic proteins, histones and acidic proteins in nucleoid bodies (NB) of specimens of different lengths: group I, 150–160 µm; group II, 190–200 µm; group III, 240–250 µm; group IV, 300–320 µm; group V, 390–420 µm; group VI, 490–520 μm: x-axis, group number; y-axis, protein content presented in arbitrary units as means (±95% confidence limits; 20 binucleoid specimens=40 nucleoids were measured for each group). Upper left frame DNA/NB content; lower right frame average volume of bacterium of the various groups (in µm3). Size of symbols are equivalent to 95% confidence limit. Volume multiply by 1000
Epulopiscium fishelsoni. Transmission electron micrographs of nucleoids: A banded and synaptic chromosomes, forming helical structures (chromomeres are partly visible); B chromosome clew in nucleoid; C chromosomes with transverse chromomeres; and D part of nucleoid with an "empty" area within (winged arrows parts of chromosomes). Scale bars: 0.5 µm (A, C, D); 8.0 µm (B)
Discussion
This study is a natural continuation of our investigations on the giant bacterium Epulopiscium fishelsoni, especially the DNA–protein interactions at various stages of its lifecycle. E. fishelsoni is not only the largest known bacterium, but also the most variable in size and volume, the largest specimens being ~354,000 µm3, which is approximately 3,000-fold greater than that of the smallest examined specimens (125.6 µm3). Such exceptional variability in bacterial size can be mediated by the storage vacuoles of certain metabolites, as observed in giant (up to 750 µm) sulfur bacteria, Thiomargarita namibiensis (Schulz and Jorgensen 2001). However, E. fishelsoni does not possess such vacuoles, and the mean DNA content per cell is proportional to the mean cell volume (Bresler et al. 1998). The presented data in Table 1 demonstrate that the DNA–basic protein interactions regulate the chromatin condensation–decondensation within the NB of E. fishelsoni, as recently also revealed for other bacteria (Sandman et al. 1998; Sharpe and Errington 1999; Azam et al. 2000; Lewis 2001), and as may also be common for eukaryotic genomes (Bendich and Drlica 2000).
The terms "polyploidy", "polyteny" and "polytene chromosomes" have been related in the past to eukaryotes only, and are usually closely associated with the giant chromosomes of Drosophila. However, polytene chromosomes have also recently been detected in the nuclei of dinoflagellates, in the macronuclei of ciliates, in several cells of Diptera, in the oocytes of amphibians, and even in the nuclei of some mammalian cells (Alberts et al. 1994; Zybina and Zybina 1996; Bhaud et al. 2000). Our results add the bacteria E. fishelsoni to this list. It is well known that polyteny is permanently associated with endoreduplication and polyploidy. Recently Bendich and Drlica (2000) listed at least seven polyploid species of bacteria, among them Escherichia coli, Methanococcus janaschii, Deinococcus radiodurans, Desulfovibrio gigas and Borrelia hermsii, with up to >10 chromosomes cell−1; Azotobacter vinlandii, with up to >100 chromosomes cell−1; and E. fishelsoni, with more than ~3,000 genome equivalents cell−1. Thus, polyploidy seems to be common for both prokaryotes and eukaryotes, possibly an ancient mechanism for genome evolution. We are currently developing a method that will allow isolation of the bacterial polytene chromosomes in order to study the number and character of amplifications. The degree of polyploidy in E. fishelsoni varied approximately 3,000-fold, the greatest variation described for bacteria (Bendich and Derlic 2000). In trying to explain why E. fishelsoni develops such a high order of polyploidy and forms numerous polytene chromosomes, we should consider the specific microenvironment of these bacteria: all known morphotypes or species (>10) within the family "Epulopiscidae" are found only in the guts of various herbivorous surgeonfishes that dwell in coral reef regions (Clements et al. 1989; Fishelson 1999) and forage on algae and detritial plant material. The gut material contains numerous natural secondary metabolites from algae and bacteria, such as ciguatoxin, in a concentration sufficiently high to be toxic to humans (Campbell et al. 1987). It is clear that this toxin was developed as protection from bacteria (Harborne 1993). A primary condition for survival in such situations is the ability of organisms to protect themselves against natural xenobiotics by numerous defense mechanisms (Harborne 1993; Saier et al. 1998). During the coevolution of symbionts, in our case the bacterium and the surgeonfish, it is reasonable to assume that selection pressure by xenobiotics induced rapid perfection of corresponding anti-xenobiotic defense mechanisms. We assume that increased polyploidy, leading towards amplification of defensive genetic possibilities (see also Alberts et al. 1994; Otto and Whitton 2000), is the result of such xenobiotic-mediated coevolution in the symbiotic E. fishelsoni.
References
Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD (1994) Molecular biology of the cell, 3rd edn. Garland, New York
Angert ER, Clements KD, Pace NR (1993) The largest bacterium. Nature 382:239–241
Angert ER, Brooks AE, Pace NR (1996) Phylogenetic analysis of Metabacterium polyspora: clues to the evolutionary origin of daughter cell production in Epulopiscium species, the largest bacterium. J Bacteriol 178:1451–1456
Azam TA, Hiraga S, Ishihama A (2000) Two types of localization of the DNA-binding proteins within Escherichia coli nucleoid. Genes Cells 5:613–626
Bendich AJ, Drlica K (2000) Prokaryotic and eukaryotic chromosomes: what's the difference. BioEssays 22:481–486
Bhaud Y, Guillelault D, Lennon J, Defacque H, Soyer-Gobillard MO, Moreau H (2000) Morphology and behavior of dinoflagellates chromosomes during the cell cycle and mitosis. J Cell Sci 113:1231–1239
Bresler V, Montgomery WL, Fishelson L, Pollak PL (1998) Gigantism in bacterium, Epulopiscium fishelsoni, correlates with complex patterns in arrangement, quantity, and segregations of DNA. J Bacteriol 180:5601–5611
Campbell B, Nakagawa LK, Kobayashi MN, Hokama Y (1987) Gambierdiscus toxicus in gut content of the surgeonfish Ctenochaetus strigosus (herbivore) in relation to toxicity. Toxicon 25:1125–1127
Clark G (1987) Staining procedures, 4th edn. Williams and Wilkins, Baltimore
Clements KD, Sutton DC, Choat JH (1989) Occurrence and characteristics of unusual protistan symbionts from surgeonfishes (Acanthuridae) of the Great Barrier Reef, Australia. Mar Biol 102:403–412
Curtis SK, Cowden RR (1985) Microfluorimetric estimates of protein associated with murine hepatocyte and thymocyte nuclei, residual structures and nuclear matrix derivates. Histochemistry 82:331–339
Fishelson L (1999) Polymorphism in gigantobacterial symbionts in the gut of surgeonfish (Acanthuridae, Teleostei). Mar Biol 133:345–351
Fishelson L, Montgomery WL, Myrberg AA (1985) Unique symbiosis in the gut of tropical herbivorous surgeonfish (Acanthuridae: Teleostei) from the Red Sea. Science 239:49–51
Geyer G (1973) Ultrahistochemistry. Fischer , Jena
Harborne JE (1993) Introduction to ecological biochemistry, 4th edn. Academic, London
Hiraga S (2000) Dynamic localization of bacterial and plasmid chromosomes. Annu Rev Genet 34:21–59
Holmes VF, Cozzarelli NR (2000) Closing the ring: links between SMC proteins and chromosome partitioning, condensation and supercoiling. Proc Natl Acad Sci USA 97:1322
Jensen RB, Shapiro L (2000) Proteins in the move: dynamic protein localization in prokaryotes. Trends Cell Biol 10:483–488
Lewis PJ (2001) Bacterial chromosome segregation. Microbiology 147:519–526
Montgomery WL, Pollak PE (1988) Epulopiscium fishelsoni n.g., n.sp., a protist of uncertain taxonomic affinities from the gut of an herbivorous reef fish. J Protozool 35:565–569
Otto SP, Whitton J (2000) Polyploidy incidence and evolution. Annu Rev Genet 34:401–437
Ruch F (1973) Quantitative determination of DNA and protein in single cells. In: Thaer AA, Sernetz M (eds) Fluorescence techniques in cell biology. Springer, Berlin, pp 89–93
Saier MH Jr, Paulsen IT, Sliwinski MK, Rao SS, Skurray RA, Nikaido H (1998) Evolutionary origins of multidrug and drug-specific efflux pumps in bacteria. FASEB J 12:265–274
Sandman K, Pereira SI, Reeve JN (1998) Diversity of prokaryotic chromosomal proteins and the origin of the nucleosome. Cell Mol Life Sci 54:1350–1364
Schulz NN, Jorgensen BB (2001) Big bacteria. Annu Rev Microbiol 55:105–137
Sharpe ME, Errington J (1999) Upheaval in the bacterial nucleoid: an active chromosome segregation mechanism. Trends Genet 15:70–74
Sharpe ME, Hauser PM, Sharpe RG, Errington J (1999) Bacillus subtilis cell cycle as studied by fluorescence microscopy: constancy of cell length at initiation of DNA replication and evidence for active nucleoid partitioning. J Bacteriol 180:547–555
Zybina EV, Zybina TG (1996) Polytene chromosomes in mammalian cells. Int Rev Cytol 165:53–119
Acknowledgements
Thanks are due to Prof. A.J. Bendich and Prof. K. Drlica (USA) for their constructive comments on a draft of the manuscript. Our gratitude goes to the Nature Protection Administration of Israel for permitting the collection of fish and to N. Paz for editing the manuscript. The constructive remarks of the reviewers are acknowledged. The experimental work in this study complied with the current laws in Israel.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Bresler, V., Fishelson, L. Polyploidy and polyteny in the gigantic eubacterium Epulopiscium fishelsoni . Marine Biology 143, 17–21 (2003). https://doi.org/10.1007/s00227-003-1055-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1055-2