Abstract
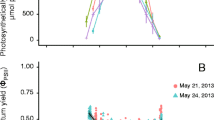
The threatened seagrass Halophila johnsonii Eiseman coexists subtidally with H. decipiens Ostenfeld in southeastern Florida, but only H. johnsonii also occurs intertidally. Pulse amplitude modulated fluorometry and fiber-optic spectrometry were used to investigate the photobiology of two populations of H. johnsonii and H. decipiens in an attempt to explain these distribution patterns. Maximum photosynthetic quantum yields (F v/F m) were measured in situ as a function of depth distribution within, and between, these two species at two sites (Jupiter Sound, 26°57′N; 80°04′W, and northern Biscayne Bay, 25°55′N; 80°07′W) along the east coast of Florida, USA, during 6–10 March 2001. Reciprocal transplants at the northern site were used to evaluate the plasticity of photosynthetic patterns and pigment absorption spectra and to gain insights into the mechanisms responsible for variations in the observed depth-distribution patterns. Subtidal-population F v/F m values were generally higher for H. johnsonii than for H. decipiens, at both sites. At the northern site, intertidal H. johnsonii had significantly lower F v/F m (0.494±0.138) than both subtidal H. johnsonii (0.696±0.045) and subtidal H. decipiens (0.668±0.048). In contrast, at the southern site intertidal H. johnsonii had the highest F v/F m (0.663±0.047) and were the largest plants. F v/F m values of subtidal plants of both species decreased when they were transplanted into shallow, intertidal beds. Correspondingly, F v/F m increased for intertidal H. johnsonii transplanted into the subtidal, 2 m deep beds. Rapid light curves indicated that H. decipiens had lower maximum relative electron transport rates (RETRmax) than did H. johnsonii. In addition, the onset of photoinhibition occurred at lower irradiances for H. decipiens (537–820 μmol photons m−2 s−1) compared to H. johnsonii (1141–2670 μmol photons m−2 s−1). RETRmax values decreased for intertidal H. johnsonii transplanted into subtidal beds, but they increased for both species when transplanted from subtidal to intertidal beds. Absorption spectra for the acetone-soluble leaf pigments of intertidal H. johnsonii exhibited a dominant peak near 345 nm; this UV peak was 30% lower for subtidal plants. Pigment absorption spectra for H. decipiens lacked the 345 nm peak and absorbances, normalized to leaf pairs, were lower across the spectrum. Our results indicate that photosynthetic tolerance to higher irradiances and presence of UV-absorbing pigments (UVP) in H. johnsonii may allow this species to exploit the shallowest waters without competition from the closely related, but UVP-lacking H. decipiens.









Similar content being viewed by others
References
Abal EG, Lorenagan N, Bowen P, Perry CJ, Udy JW, Dennison WC (1994) Physiological and morphological responses of the seagrass Zostera capricorni Aschers, to light intensity. J Exp Mar Biol Ecol 178:113–129
Beer S, Vilenkin B, Weil A, Veste M, Susel L, Eshel A (1998) Measuring photosynthetic rates in seagrasses by pulse amplitude modulated (PAM) fluorometry. Mar Ecol Prog Ser 174:293–300
Beer S, Björk M, Gademann R, Ralph P (2001) Measurements of photosynthetic rates in seagrasses. In: Short FT, Coles RG (eds) Global seagrass research methods. Elsevier, Amsterdam, pp 183–198
Björk M, Uku J, Weil A, Beer S (1999) Photosynthetic tolerances to dessication of tropical intertidal seagrasses. Mar Ecol Prog Ser 191:121–126
Dawes CJ, Lobban CS, Tomasko DA (1989) A comparison of the physiological ecology of the seagrasses Halophila decipiens Ostenfeld and H. johnsonii Eiseman from Florida. Aquat Bot 33:149–154
Dawson SP, Dennison WC (1996) Effects of ultraviolet and photosynthetically active radiation on five seagrasses. Mar Biol 125:629–638
den Hartog C (1970) The seagrasses of the world. North-Holland, Amsterdam
Detras Y, Armstrong RA, Connelly XM (2001) Ultraviolet-induced responses in two species of climax tropical marine macrophytes. J Photochem Photobiol B Biol 62:55–66
Durako MJ, Kunzelman JI (2002) Photosynthetic characteristics of Thalassia testudinum measured in situ by pulse-amplitude modulated (PAM) fluorometry: methodological and scale-based considerations. Aquat Bot 73:173–185
Eiseman NJ, McMillan C (1980) A new species of seagrass, Halophila johnsonii, from the Atlantic coast of Florida. Aquat Bot 9:15–19
Federal Register (1998) Endangered and threatened species: threatened status for Johnson's seagrass. 63:49035–49041
Franklin LA, Seaton GGR, Lovelock CE, Larkum AWD (1996) Photoinhibition of photosynthesis on a coral reef. Plant Cell Environ 19:825–836
Gorbunov MY, Kolber ZS, Lesser MP, Falkowski PG (2001) Photosynthesis and photoprotection in symbiotic corals. Limnol Oceanogr 46:75–85
Hader DP, Kumar HD, Smith RC, Worrest RC (1998a) Effects on aquatic ecosystems. J Photochem Photobiol B Biol 46:53–68
Hader DP, Lebert M, Figueroa FL, Jimanez C, Viaegla B, Perez-Rodriguez E (1998b) Photoinhibition in Mediterranean macroalgae by solar radiation measured on site by PAM fluorescence. Aquat Bot 61:225–236
Heidelbaugh WS, Hall LM, Kenworthy WJ, Whitfield P, Virnstien RW, Morris LJ, Hannisak MD (1999) Reciprocal transplanting of the threatened seagrass, Halophila johnsonii (Johnson's seagrass) in the Indian River lagoon, Florida. In: Bortone SA (ed) Seagrasses: monitoring, ecology, physiology, and management. CRC Press, Boca Raton, Fla., pp 197–210
Jassby AD, Platt T (1976) Mathematical formulations of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547
Jeffrey SW, Humphrey GF (1975) New spectrophotometric equations for determining chlorophylls a, b, c 1, and c 2 in higher plants, algae, and natural phytoplankton. Biochem Physiol Pflanz 167:191–194
Kenworthy WJ (1993) The distribution, abundance and ecology of Halophila johnsonii Eiseman in the lower Indian River, Florida. Final Rept Office of Protected Species, National Marine Fisheries Service, Silver Spring, Md.
Kenworthy WJ (2000) The role of sexual reproduction in maintaining populations of Halophila decipiens: implications for the biodiversity and conservation of tropical seagrass ecosystems. Pac Conserv Biol 5:260–268
Krause GH (1991) Photoinhibition of photosynthesis. An evaluation of damaging and protective mechanisms. Physiol Plant 74:566–574
Major KM, Dunton KH (2002) Variations in light-harvesting characteristics of the seagrass, Thalassia testudinum: evidence for photoacclimation. J Exp Mar Biol Ecol 275:173–189
McMillan C, Zapata O, Escobar L (1980) Sulphated phenolic compounds in seagrasses. Aquat Bot 8:267–278
Ralph PJ, Burchett MD (1995) Photosynthetic responses of the seagrass Halophila ovalis (R. Br.) Hook. f. to high irradiance stress, using chlorophyll a fluorescence. Aquat Bot 51:55–66
Ralph PJ, Gademann R, Dennison WC (1998) In situ seagrass photosynthesis measured using a submersible, pulse-amplitude modulated fluorometer. Mar Biol 132:367–373
Schwarz AM, Bjark M, Buluda T, Mtolera M, Beer S (2000) Photosynthetic utilisation of carbon and light by two tropical seagrass species measured in situ. Mar Biol 137:755–761
Sinha RP, Klisch M, Graniger A, Hader DP (1998) Ultraviolet-absorbing/screening substances in cyanobacteria, phytoplankton and macroalgae. J Photochem Photobiol B Biol 47:83–94
Sinha RP, Klisch M, Hader DP (1999) Induction of a mycosporine-like amino acid (MAA) in the rice-field cyanobacterium Anabaena sp. by UV radiation. J Photochem Photobiol B Biol 52:59–64
Trocine RP, Rice JD, Wells GN (1981) Inhibition of seagrass photosynthesis by ultraviolet-B radiation. Plant Physiol (Rockv) 68:74–81
Van Kooten O, Snel JFH (1990) The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosyn Res 25:147–150
Virnstein RW, Morris LJ, Miller JD, Miller-Meyers R (1997) Distribution and abundance of Halophila johnsonii in the Indian River lagoon. District technical memo no. 24, St. Johns River Water Managment District
Yakoleva IM, Titlyanov EA (2001) Effect of high visible and UV irradiance on subtidal Chondrus crispus: stress, photoinhibition and protective mechanisms. Aquat Bot 71:47–61
Acknowledgements
This project was supported by the National Oceanic and Atmospheric Administration, Recover Protected Species Program, Center for Coastal Fisheries and Habitat Research, National Ocean Service, Beaufort, N.C.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P. Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Durako, M.J., Kunzelman, J.I., Kenworthy, W.J. et al. Depth-related variability in the photobiology of two populations of Halophila johnsonii and Halophila decipiens . Marine Biology 142, 1219–1228 (2003). https://doi.org/10.1007/s00227-003-1038-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1038-3