Abstract
In this study, the influence of dielectric barrier discharges at atmospheric pressure on maple sapwood was analyzed. Three common discharge types, a coplanar surface barrier discharge (CSBD), a direct dielectric barrier discharge and a jet discharge/remote plasma, were realized by the same electrodes and power supply. In general, plasma treatments are known to modify various surface properties leading to improved adhesion. For resins such as urea formaldehyde, the curing behavior is dependent on the pH. Therefore, the change of the surface pH value of maple is measured. As the main product of plasma-generated nitrogen oxides, the nitrate concentrations and consequently the nitric acid concentrations of the surfaces were determined. A significant pH reduction from about pH 5.6 to pH 4.6 was reached after a 30 s plasma treatment. An increase in the nitrate concentration over the plasma treatment duration was measured for all three discharges with a maximum of 6.6 mg/L for the CSBD, which corresponds to a concentration of 8.25 mg per m2 wooden surface.
Similar content being viewed by others
Introduction
Wood is a traditional and continuously important material which has a comparatively low cost, a renewable and sustainable production, and a balanced relation of weight and mechanical strength (Risbrudt 2005). Further advantages of natural wood are the simple processing and low expansion due to thermal changes (Weatherwax and Stamm 1956). However, wood has also negative properties like low dimensional stability due to moisture content changes (Stamm 1964), changes in appearance due to weathering (Williams 2005; Evans 2009) and partially low resistance against fungi and insects (Ibach 2005). Objectives of the research are to minimize the negative properties of natural wood to give wood a wide field of applications. Therefore, solid wood is either coated by different coating systems (Hill 2006; Kielmann and Mai 2016), thermally and chemically modified, or wood-based panels are produced from wood chips, veneers or fibers. Wood-based panels have an increased dimensional stability, reduced anisotropy and higher homogeneity compared to solid wood (Dunky and Niemz 2002). In the wood-based panel industry, mainly formaldehyde-based resins (urea-, phenol- and melamine–formaldehyde resins) and isocyanates are applied (Stamm et al. 1962; Dunky 1998; Papadopoulos et al. 2002; Franke et al. 2016). The weak point of the system (coated solid wood and wood-based panels) is the adhesive strength, which is defined as the ability to hold two materials together under a given set of conditions (Frihart 2006). The adhesive strength is influenced by various factors like wood surface roughness, wood surface energy, flow of the adhesive across and into the wood pores/wood cell walls, adhesive viscosity and processing pressure. Further factors are the composition of the material with regard to the extractives and the functional groups on the surface as well as the pH value of the wood surface (Cheng and Sun 2006; Frihart 2006).
Plasma treatment of wood is known to lower the water contact angle and increase the wettability, surface free energy and the adhesion strength (Podgorski et al. 2000; Aydin and Demirkir 2010; Busnel et al. 2010; Acda et al. 2012; Viöl et al. 2013; Wascher et al. 2014a, b; Prégent et al. 2015a, b; Altgen et al. 2016; Hünnekens et al. 2016; Peters et al. 2017). During plasma treatment, radical species are placed on the surfaces (Hardy et al. 2015) and can replace or increase the effect of sanding (Evans et al. 2007; Wolkenhauer et al. 2009; Prégent et al. 2015b).
The influence of the pH is shown in studies like for example Johns and Niazi (1980) where the dependence of the urea formaldehyde resin gelation time on the pH value and the buffering capacity of wood is determined.
A pH reduction after a plasma treatment was measured using a surface pH electrode (Odrášková et al. 2008; Helmke et al. 2009; Wascher et al. 2014b). However, the increased wettability of wooden surfaces leads to absorption of test liquid into the material after plasma treatments, which causes a systematical error of measurements with pH surface electrodes. Therefore, this study compares soaking and rinsing sample preparations.
The field of wooden-surface plasma treatment at atmospheric pressure is dominated by dielectric barrier discharges due to the low thermal impact. The dielectric barrier discharges are mainly used in the setups as direct volume barrier discharge (Topala and Dumitrascu 2007; Acda et al. 2012; Altgen et al. 2016; Avramidis et al. 2016; Hünnekens et al. 2016; Peters et al. 2017), coplanar surface barrier discharge (Odrášková et al. 2008; Černák et al. 2009) and remote plasma (Busnel et al. 2010; Riedl et al. 2014; Hardy et al. 2015; Prégent et al. 2015a).
In this study, the influence of atmospheric-pressure plasma treatment on chemical properties such as pH value and the nitrate concentration was measured on maple as a wooden material with a homogenous structure and a relatively low extractive content. The extractives can be transported toward the surface by a plasma treatment and decrease the wettability (Evans et al. 2007), so that higher energies are needed. The influences of three dielectric barrier discharges—a direct dielectric barrier discharge (DDBD), a coplanar surface barrier discharge (CSBD) and a remote plasma (RP)—are compared. All three discharges are based on the same electrodes and power supply and adjusted to the same power to minimize the influence of different powers, although the influence of different power densities still exists.
Materials and methods
Setup
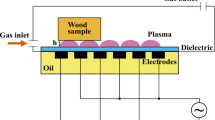
For the plasma treatment of wood, plasma sources based on the principal of the dielectric barrier discharge (DBD) were used. All three experimental setups were based on the same electrodes consisting of 250-mm-long ceramic (Al2O3) square tubes of 2.5 mm thickness, filled with bronze and air cooling. For the CSBD (Fig. 1), the first electrode was connected to the high voltage and the second electrode was grounded. The distance between the electrodes was 15 mm, including an additional glass barrier of 3 mm. The discharge gap toward the sample was 0.5 mm. In contrast to other CSBD with a completely filled gap, the gap of this CSBD contains air and an additional glass barrier as dielectric material. The RP (Fig. 2) was configured in the same electrical manner but without an additional glass barrier and with gaps of 2 mm between the electrodes as well as between the electrodes and the sample. For the DDBD (Fig. 3), distances were the same as for the RP. The DDBD differed from the RP in that both tubular electrodes were contacted to the high voltage and a grounded aluminum electrode, covered by 4 mm glass, was below the samples.
For all three setups, the same power supply was used. The power supply was pulsed with a frequency of 15 kHz with alternating voltage pulses of 27 µs duration. For neglecting the thermal impact (surface temperatures after 30 s plasma treatment below 320 K, see Table 1) and the possibility of stationary filaments, 1 s of plasma treatment was followed by a pause of 1 s. As process gas, 120 L/min dried compressed air with a constant amount of humidity (less than 6.5%) was used.
A physical characterization of the three filamentary dielectric barrier discharges at atmospheric pressure is shown in Peters et al. (2017); a summary of the relevant data is in Table 1.
Materials
Solid sapwood of maple (Acer spec.) with a mass density of (633.6 ± 3.7) kg m−3 was cut into pieces of (80 × 50 × 4) mm3. The tests were performed on the radial surface.
Prior to testing, the samples were stored in a climate chamber at 20 °C/65% RH for 2 weeks.
Plasma treatment and sample preparation
For the measurements, extracts of the whole wood surfaces (80 × 50) mm2 were created by rinsing or soaking the surface with 5 ml low-level ionic strength adjuster (ISA) solution. The low-level ISA solution for nitrate determinations consists of 20% ISA solution Nitrate (Mettler-Toledo GmbH, Gießen, Germany) and 80% deionized water.
The soaking times were 10 and 60 s. For the rinsing method, the surfaces were rinsed repeatedly three times with the same 5 ml low-level ISA solution. In this way, three methods of sample preparation were compared.
The plasma treatment time was chosen as in Peters et al. (2017): 0 s as reference, 1, 5, 10 and 30 s.
For each discharge type, three samples per plasma treatment time and sample preparation were tested.
pH- and nitrate measurements
The pH of the extracts was measured with a pH-sensitive electrode, InLab Water Go (Mettler-Toledo GmbH, Gießen, Germany). The nitrate concentrations of the same extracts were determined with an ion selective nitrate electrode, perfectION comb NO3 (Mettler-Toledo GmbH, Gießen, Germany) with a detection limit of 0.1 mg/L. For nitrate measurements, the low-level calibration was performed according to the manufacturer’s manual. Before each measuring sequence, the pH electrode was calibrated using a pH 4.0 and pH 7.0 standard buffer solution.
Titration
For the titration, maple samples with the dimension of (250 × 50 × 4) mm3 were soaked in or rinsed off with 15 ml deionized water. The resulting extracts of two samples were combined. Two 10 ml samples were titrated to determine either the acid or the alkaline buffer capacity. For the titration, 5 mM HCl and NaOH in deionized water were used. The values are the mean of two titrations. HCl and NaOH solutions were added in steps until a pH of 3 or 7 was exceeded (according to Johns and Niazi 1980). The acid equivalent, also called acid buffering capacity, is defined as the amount of NaOH solution required to raise the pH to pH of 7.0 per volume. Consequently, alkaline equivalent or alkaline buffering is the amount of HCl solution necessary to reach the pH 3.0 per volume. The total buffering capacity is the sum of both buffering capacities.
An additional titration was done for the 60-s soaking extracts of the 30 s plasma-treated wood samples in low-level ISA solution. For that, the three extracts for each discharge type were combined and aliquots of 5 ml were used.
Statistical analysis
All data are given as mean ± standard deviation calculated from at least three independent sample treatments unless noted otherwise. Statistical analyses were performed with IBM SPSS Statistics version 24 (IBM, Armonk, USA).
Differences between data groups were evaluated according to the three between-subjects factors discharge type, extract type and treatment time, using a multivariate analysis based on generalized linear models. For paired post hoc tests with a significance level of α = 0.05, the Tukey correction of p values was employed. The influence of all three between-subjects factors on nitrate concentration and pH was determined to be statistically significant.
In general, apart from 1 s plasma treatments, all changes in pH and nitrate were significant compared to reference samples.
Results and discussion
The pH changes dependent on treatment time are shown in Fig. 4 for CSBD, in Fig. 5 for DDBD, and in Fig. 6 for RP. For all three discharges, the initial pH value of about 5.6 was in the same range as presented in Johns and Niazi (1980) and is reduced asymptotically by 0.4–1.0 after 30 s of plasma treatment. The method of sample preparation (rinsed, 10- or 60-s soaking) also influences the size of the effect. The 60-s soaking method shows the smallest change of pH, while the pH of rinsed samples decreases the most.
The acid which is most likely to be responsible for the pH decrease is nitric acid (HNO3) (Dorai and Kushner 2003). As shown in Fig. 7, nitric acid can be formed by various reactions of nitric oxide and nitrogen oxide, which are common species in plasma discharges (Stefanovic et al. 2001; Kogelschatz 2003; Peters et al. 2016). The process gas is compressed dried air, thus the source for humidity can be the surrounding air and the moisture of the maple samples.
To verify the generation of nitric acid, nitrate concentrations of the samples were analyzed. The amount of nitrogen in wooden material is less than 0.2 wt% (Keller and Nussbaumer 1993). Thus, the error by nitrate originating from the wood is expected to be negligible.
For the references (0 s), nitrate concentrations of less than 1 mg/L were measured. The plasma treatment increased the nitrate concentrations by 1–6 mg/L (Figs. 8, 9, 10). The highest concentrations were determined for the 60-s soaking samples and the lowest for the rinsed ones.
Increase in nitrate concentrations for all three extract types after CSBD plasma treatment. The maximum value is detected in the 60-s soaking sample after 30 s treatment with a concentration of 6.6 mg/L. The concentration for the rinsed sample is 2.1 mg/L lower; the 10-s soaking sample falls in between
This relation between nitrate concentration and extract type is the opposite of that for the pH change.
Thus, the sample preparation seems to have a significant influence on the measured pH and the nitrate concentration. While the pH value decreases less with a longer sample soaking time, the nitrate concentration increases. A possibility for observing a smaller pH change together with higher nitrate concentrations is the dissolution of pH-buffering substances from the wood.
For this reason, the acid and alkaline buffer capacities for samples without plasma treatment, prepared with deionized water, were measured. The resulting data are shown in Table 2. As expected, it is lowest for deionized water used as reference and highest for the 60-s soaking samples. Thus, the increased buffer capacity for the 60-s soaking samples explains the lower effect of the nitrate concentration on the pH.
To determine the differences between the discharge types, the mean of the different extract types was calculated. The means showed nearly the same pH for CSBD and DDBD, but a difference in the nitrate concentration. So, the relation between the measured nitrate concentrations and the pH is shown in Fig. 11. The fits show a difference, especially between the CSBD and the RP. Therefore, the buffering capacity was measured for the plasma-treated samples.
The acid buffering capacity determined for the 60-s soaking extracts of untreated and plasma-treated wood samples (Table 3) shows a difference for CSBD and DDBD. While the acid buffering capacity for CSBD-treated extract is roughly the same as for the untreated one and the RP-treated sample, the acid buffering capacity for the extract of the DDBD treatment is markedly lower.
This divergence can explain the same pH change with lower nitrate concentrations. The buffering capacity is caused by weak acids, such as acetic acid (Risholm-Sundman et al. 1998), originating from the wood. A degradation of these weak acids is plausible for the DDBD due to the depth effect and direct electron bombardment (Meiners et al. 2012; Wascher et al. 2014b). The RP shows only half the pH reduction seen for CSBD and DDBD and about half of the CSBD nitrate concentrations. This is consistent with the same acid buffering capacity for CSBD and RP. Differences between the presented discharge types in surface power density, discharge volume power density and the gas temperatures (see Table 1) as well as the remote character of the RP lead to changes in the nitric acid production. Dorai and Kushner (2003) showed simulations of densities of gas phase species depending on the energy deposition and gas temperature, especially for the production of HNO3. Although the simulated range of energy deposition is much lower than the energies applied by the three discharge types in this study, the tendency can be applied. The energies per discharge volume for the CSBD, DDBD and RP are related as follows: \( \left( {E/V} \right)_{\text{CSBD}} \approx 3\left( {E/V} \right)_{\text{RP}} \approx 8\left( {E/V} \right)_{\text{DDBD}} \). Dorai and Kushner (2003) showed an increase in the HNO3 density from 3 to 14 × 1015 cm−3 for a range of the energy deposition from 1250 to 3750 J/L. This rate could not be measured for the presented discharges. Nevertheless, the difference in nitrate concentrations for CSBD and DDBD might be explained by this behavior. Also the influence of the setups and the energies on the production of nitrogen oxides has to be investigated separately. The lower concentration for the RP results from the remote character and the consequently lower density of plasma species on the surface.
Conclusion
The plasma treatment of all three discharges leads to a significant reduction in the pH of wooden surfaces. On maple, reductions of up to 1.0 are possible depending on the discharge type and the amount of buffer dissolved. In general, the pH of wooden surfaces is lowered by air plasma treatment and has to be taken into account for adhesion processes with plasma pretreatment.
The comparison of the three discharges showed the same pH change for CSBD and DDBD and half of the change for RP. The pH reduction results from plasma-generated acid, mainly nitric acid. Nitrate concentrations up to 6.6 mg/L, were measured. This corresponds to a nitrate deposition of 8.25 mg per m2 wooden surface. For DDBD, a reduction in the acid buffering capacity is shown. The depth effect and electron bombardment are likely to be responsible for this degradation.
References
Acda MN, Devera EE, Cabangon RJ, Ramos HJ (2012) Effects of plasma modification on adhesion properties of wood. Int J Adhes Adhes 32:70–75. https://doi.org/10.1016/j.ijadhadh.2011.10.003
Altgen D, Avramidis G, Viöl W, Mai C (2016) The effect of air plasma treatment at atmospheric pressure on thermally modified wood surfaces. Wood Sci Technol 50:1227–1241. https://doi.org/10.1007/s00226-016-0856-7
Avramidis G, Wascher R, Militz H, Viöl W (2016) Impact of air-plasma treatment at atmospheric pressure on wood and wood extractives. Int Wood Prod J 7:76–79. https://doi.org/10.1080/20426445.2016.1162429
Aydin I, Demirkir C (2010) Activation of spruce wood surfaces by plasma treatment after long terms of natural surface inactivation. Plasma Chem Plasma Process 30:697–706. https://doi.org/10.1007/s11090-010-9244-5
Busnel F, Blanchard V, Prégent J, Stafford L, Riedl B, Blanchet P, Sarkissian A (2010) Modification of sugar maple (Acer saccharum) and black spruce (Picea mariana) wood surfaces in a dielectric barrier discharge (DBD) at Atmospheric pressure. J Adhes Sci Technol 24:1401–1413. https://doi.org/10.1163/016942410X501007
Černák M, Černáková L, Hudec I, Kováčik D, Zahoranová A (2009) Diffuse Coplanar Surface Barrier Discharge and its applications for in-line processing of low-added-value materials. Eur Phys J Appl Phys 47:22806. https://doi.org/10.1051/epjap/2009131
Cheng E, Sun X (2006) Effects of wood-surface roughness, adhesive viscosity and processing pressure on adhesion strength of protein adhesive. J Adhes Sci Technol 20:997–1017. https://doi.org/10.1163/156856106777657779
Dorai R, Kushner MJ (2003) A model for plasma modification of polypropylene using atmospheric pressure discharges. J Phys D Appl Phys 36:666. https://doi.org/10.1088/0022-3727/36/6/309
Dunky M (1998) Urea–formaldehyde (UF) adhesive resins for wood. Int J Adhes Adhes 18:95–107. https://doi.org/10.1016/S0143-7496(97)00054-7
Dunky M, Niemz P (2002) Holzwerkstoffe und Leime: Technologie und Einflussfaktoren (Wood-based products and adhesives: technology and influencing factors). Springer, Berlin (In German)
Evans PD (2009) Review of the weathering and photostability of modified wood. Wood Mater Sci Eng 4:2–13. https://doi.org/10.1080/17480270903249391
Evans PD, Ramos M, Senden T (2007) Modification of wood using a glow-discharge plasma derived from water. In: Hill CAS, Jones D, Militz H, Ormondroyd GA (eds) proceedings of the third European conference on wood modification, pp 123–132
Franke T, Lenz C, Herold N, Pfriem A (2016) Investigations on phenol/formaldehyde impregnation of beech veneer for improved plasticization and form stability. In: Eberhardsteiner J, Winter W, Fadai A (eds) WCTE 2016 e-book: containing all full papers submitted to the world conference on timber engineering (WCTE 2016), August 22–25, 2016, Vienna, Austria
Frihart CR (2006) Wood Structure and Adhesive Bond Strength. In: Stokke DD, Groom LH (eds) Characterization of the cellulosic cell wall, 1st edn. Blackwell Pub, Ames
Hardy J-M, Levasseur O, Vlad M, Stafford L, Riedl B (2015) Surface free radicals detection using molecular scavenging method on black spruce wood treated with cold, atmospheric-pressure plasmas. Appl Surf Sci 359:137–142. https://doi.org/10.1016/j.apsusc.2015.10.062
Helmke A, Hoffmeister D, Mertens N, Emmert S, Schuette J, Vioel W (2009) The acidification of lipid film surfaces by non-thermal DBD at atmospheric pressure in air. New J Phys 11:115025. https://doi.org/10.1088/1367-2630/11/11/115025
Hill CAS (2006) Wood modification: Chemical, thermal and other processes. Wiley series in renewable resources. Wiley, Chichester
Hünnekens B, Peters F, Avramidis G, Krause A, Militz H, Viöl W (2016) Plasma treatment of wood–polymer composites: a comparison of three different discharge types and their effect on surface properties. J Appl Polym Sci. https://doi.org/10.1002/app.43376
Ibach RE (2005) Biological Properties. In: Rowell RM (ed) Handbook of wood chemistry and wood composites. CRC Press, Boca Raton
Johns WE, Niazi KA (1980) Effect of pH and buffering capacity of wood on the gelation time of urea-formaldehyde resin. Wood Fiber Sci 12:255–263
Keller R, Nussbaumer T (1993) Bestimmung des Stickstoffgehalts von Holz und Holzwerkstoffen mittels Oxidation und Chemilumineszenz-Detektion von Stickstoffmonoxid (Determination of the nitrogen content in wood by combustion and chemilumescent detection of nitric oxides). Holz Roh- Werkst. 51:21–26. https://doi.org/10.1007/BF02615372 (In German)
Kielmann BC, Mai C (2016) Natural weathering performance and the effect of light stabilizers in water-based coating formulations on resin-modified and dye-stained beech-wood. J Coat Technol Res 13:1065–1074. https://doi.org/10.1007/s11998-016-9818-0
Kogelschatz U (2003) Dielectric-barrier discharges: their history, discharge physics, and industrial applications. Plasma Chem Plasma Process 23:1–46. https://doi.org/10.1023/A:1022470901385
Manion JA, Huie RE, Levin RD, Burgess DR Jr, Orkin VL, Tsang W, McGivern WS, Knyazev VD, Atkinson DB, Chai E, Tereza AM, Lin C-Y, Allison TC, Mallard WG, Westley F, Herron JT, Hampson RF, Frizzell DH (2017) NIST chemical kinetics database: NIST standard reference database 17. National Institute of Standards and Technology, Gaithersburg, pp 20899–28320
Meiners A, Ohms G, Leck M, Vetter U, Abel B (2012) Modifying glass fiber size by plasma treatment. J Adhes Sci Technol 26:1611–1627. https://doi.org/10.1163/156856111X618443
Odrášková M, Ráhel’ J, Zahoranová A, Tiňo R, Černák M (2008) Plasma activation of wood surface by diffuse coplanar surface barrier discharge. Plasma Chem Plasma Process 28:203–211. https://doi.org/10.1007/s11090-007-9117-8
Papadopoulos AN, Hill CAS, Traboulay E, Hague JRB (2002) Isocyanate resins for particleboard: PMDI vs EMDI. Eur J Wood Prod 60:81–83. https://doi.org/10.1007/s00107-001-0275-8
Peters F, Hirschberg J, Mertens N, Wieneke S, Viöl W (2016) Comparison of nitric oxide concentrations in μs- and ns-atmospheric pressure plasmas by UV absorption spectroscopy. Plasma Sci Technol 18:406. https://doi.org/10.1088/1009-0630/18/4/13
Peters F, Hünnekens B, Wieneke S, Militz H, Ohms G, Viöl W (2017) Comparison of three dielectric barrier discharges regarding their physical characteristics and influence on the adhesion properties on maple, high density fiberboards and wood plastic composite. J Phys D Appl Phys. https://doi.org/10.1088/1361-6463/aa8fad
Podgorski L, Chevet B, Onic L, Merlin A (2000) Modification of wood wettability by plasma and corona treatments. Int J Adhes Adhes 20:103–111. https://doi.org/10.1016/S0143-7496(99)00043-3
Prégent J, Vandsburger L, Blanchard V, Blanchet P, Riedl B, Sarkissian A, Stafford L (2015a) Determination of active species in the modification of hardwood samples in the flowing afterglow of N2 dielectric barrier discharges open to ambient air. Cellulose 22:811–827. https://doi.org/10.1007/s10570-014-0496-8
Prégent J, Vandsburger L, Blanchard V, Blanchet P, Riedl B, Sarkissian A, Stafford L (2015b) Modification of hardwood samples in the flowing afterglow of N2–O2 dielectric barrier discharges open to ambient air. Cellulose 22:3397–3408. https://doi.org/10.1007/s10570-015-0736-6
Riedl B, Angel C, Prégent J, Blanchet P, Stafford L (2014) Effect of wood surface modification by atmospheric-pressure plasma on waterborne coating adhesion. BioResources 9:4908–4923. https://doi.org/10.15376/biores.9.3.4908-4923
Risbrudt CD (2005) Wood and Society. In: Rowell RM (ed) Handbook of wood chemistry and wood composites. CRC Press, Boca Raton
Risholm-Sundman M, Lundgren M, Vestin E, Herder P (1998) Emissions of acetic acid and other volatile organic compounds from different species of solid wood. Holz Roh- Werkst 56:125–129. https://doi.org/10.1007/s001070050282
Stamm AJ (1964) Wood and cellulose science. Ronald Press Co, New York
Stamm AJ, Seborg RM, Forest Products Laboratory (1962) Forest Products laboratory resin-treated wood (IMPREG). Forest Products Laboratory, Forest Service, U.S. Department of Agriculture
Stefanovic I, Bibinov NK, Deryugin AA, Vinogradov IP, Napartovich AP, Wiesemann K (2001) Kinetics of ozone and nitric oxides in dielectric barrier discharges in O2/NOx and N2/O2/NOx mixtures. Plasma Sources Sci Technol 10:406. https://doi.org/10.1088/0963-0252/10/3/303
Topala I, Dumitrascu N (2007) Dynamics of the wetting process on dielectric barrier discharge (DBD)-treated wood surfaces. J Adhes Sci Technol 21:1089–1096. https://doi.org/10.1163/156856107782105936
Viöl W, Avramidis G, Militz H (2013) Plasma Treatment of Wood. In: Rowell RM (ed) Handbook of wood chemistry and wood composites, 2nd edn. CRC Press, Boca Raton, pp 627–658
Wascher R, Schulze N, Avramidis G, Militz H, Viöl W (2014a) Increasing the water uptake of wood veneers through plasma treatment at atmospheric pressure. Eur J Wood Prod 72:685–687. https://doi.org/10.1007/s00107-014-0815-7
Wascher R, Avramidis G, Vetter U, Damm R, Peters F, Militz H, Viöl W (2014b) Plasma induced effects within the bulk material of wood veneers. Surf Coat Technol 259:62–67. https://doi.org/10.1016/j.surfcoat.2014.07.005
Weatherwax RC, Stamm AJ (1956) The coefficients of thermal expansion of wood and wood products. Madison, Wis.: U.S. Dept. of Agriculture, Forest Service, Forest Products Laboratory
Williams RS (2005) Weathering of Wood. In: Rowell RM (ed) Handbook of wood chemistry and wood composites. CRC Press, Boca Raton
Wolkenhauer A, Avramidis G, Hauswald E, Militz H, Viöl W (2009) Sanding vs. plasma treatment of aged wood: a comparison with respect to surface energy. Int J Adhes Adhes 29:18–22. https://doi.org/10.1016/j.ijadhadh.2007.11.001
Acknowledgements
This work was funded by the Lower Saxony Ministry of Science and Culture through the program “Materialforschung Holz” and by the German Federal Ministry of Education and Research (BMBF) through the research project “PlaNaWood” (Grant No. 03X5519B) under the supervision of Dr. Karen Otten in Jülich, Germany. The authors thank Roger Skarsten, HAWK, for proofreading of the manuscript and Bernd Lütkemeier, Georg August University of Goettingen.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Peters, F., Gelker, M., Fleckenstein, M. et al. Decrease of the surface pH of maple and the production of nitrate by three pulsed dielectric barrier discharges. Wood Sci Technol 52, 1495–1510 (2018). https://doi.org/10.1007/s00226-018-1036-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-018-1036-8