Abstract
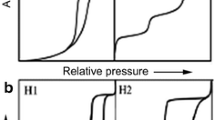
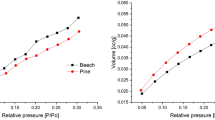
Wood cell wall pores are essential for understanding nanostructure and subsequent application to wood processing and new product design. CO2 and N2 sorption isotherms were used to explore nanopores in dried cell walls of Douglas-fir, aspen and western red cedar heartwood specimens. The total cell wall pore volume was estimated as sum of detected micropore volume from the CO2 isotherm and the volume of pores that are less than 10 nm from the N2 isotherm. The estimated pore volumes from the gas sorption method were statistically lower than those obtained from the classical pycnometer method using water and white mineral oil as replacing liquids. Large open hysteresis was observed in CO2 sorption isotherms. Further pore distribution analysis assigned the detected 10–36 nm pores to those in pit membranes. Extraction of western red cedar heartwoods largely affected the detected 0.4–0.6 nm pores, which strongly suggests penetration of extractives into cell walls. Despite the exploratory nature of this study on western red cedar, CO2 sorption analysis shows great potential in exploring micro-distribution of extractives.




Similar content being viewed by others
References
Ahlgren PA (1970) Chlorite delignification of spruce wood. Dissertation, McGill University
Babu DJ, Lange M, Cherkashinin G, Issanin A, Staudt R, Schneider JJ (2013) Gas adsorption studies of CO2 and N2 in spatially aligned double-walled carbon nanotube arrays. Carbon 61:616–623
Busse-Wicher M, Gomes TC, Tryfona T, Nikolovski N, Stott K, Grantham NJ, Bolam DN, Skaf MS, Dupree P (2014) The pattern of xylan acetylation suggests xylan may interact with cellulose microfibrils as a twofold helical screw in the secondary plant cell wall of Arabidopsis thaliana. Plant J 79(3):492–506
Chang SS, Quignard F, Alméras T, Clair B (2015) Mesoporosity changes from cambium to mature tension wood: a new step toward the understanding of maturation stress generation in trees. New Phytol 205(3):1277–1287
Clair B, Gril J, Di Renzo F, Yamamoto H, Quignard F (2008) Characterization of a gel in the cell wall to elucidate the paradoxical shrinkage of tension wood. Biomacromol 9(2):494–498
Côté WA (1963) Structural factors affecting the permeability of wood. J Polym Sci 2(1):231–242
Ding SY, Liu YS, Zeng Y, Himmel ME, Baker JO, Bayer EA (2012) How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 338(6110):1055–1060
Flournoy DS, Kirk TK, Highley TL (1991) Wood decay by brown-rot fungi: changes in pore structure and cell wall volume. Holzforschung 45(5):383–388
Harris DC (2003) Quantitative chemical analysis. W.H. Freeman, New York
Hill CAS, Papadopoulos AN (2001) A review of methods used to determine the size of the cell wall microvoids. J Inst Wood Sci 15(6):337–345
Hillis WE (1987) Heartwood and tree exudates. Springer, New York
Jansen S, Choat B, Pletsers A (2009) Morphological variation of intervessel pit membranes and implications to xylem function in angiosperms. Am J Bot 96(2):409–419
Jansen S, Lamy JB, Burlett R, Cochard H, Gasson P, Delzon S (2012) Plasmodesmatal pores in the torus of bordered pit membranes affect cavitation resistance of conifer xylem. Plant Cell Environ 35(6):1109–1120
Kellogg RM, Wangaard FF (1969) Variation in the cell-wall density of wood. Wood Fiber Sci 1(3):80–204
Kojiro K, Furuta Y, Ishimaru Y (2008a) Influence of heating and drying history on micropores in dry wood. J Wood Sci 54(3):202–207
Kojiro K, Furuta Y, Ohkoshi M, Ishimaru Y, Yokoyama M, Sugiyama J, Kawai S, Mitsutani T, Ozaki H, Sakamoto M, Imamura M (2008b) Changes in micropores in dry wood with elapsed time in the environment. J Wood Sci 54(6):515–519
Kojiro K, Miki T, Sugimoto H, Nakajima M, Kanayama K (2010) Micropores and mesopores in the cell wall of dry wood. J Wood Sci 56(2):107–111
Koumoutsakos A, Avramidis S (2002) Mass transfer characteristics of western hemlock and western red cedar. Holzforschung 56(2):185–190
Kulasinski K, Guyer RA (2016) Quantification of nanopore networks: application to amorphous polymers. J Phys Chem C 120(49):28144–28151
Kulasinski K, Guyer R, Keten S, Derome D, Carmeliet J (2015) Impact of moisture adsorption on structure and physical properties of amorphous biopolymers. Macromolecules 48(8):2793–2800
Kuo ML, Arganbright DG (1980) Cellular distribution of extractives in redwood and incense cedar—part I. Radial variation in cell-wall extractive content. Holzforschung 34(1):17–22
Li G, Wang Z (2013) Microporous polyimides with uniform pores for adsorption and separation of CO2 gas and organic vapors. Macromolecules 46(8):3058–3066
Lowell S, Shield JE, Thomas MA, Thommes M (2004) Characterization of porous solids and powders: surface area, pore size and density. Kluwer, Boston
Morris PI, Stirling R (2012) Western red cedar extractives associated with durability in ground contact. Wood Sci Technol 46(5):991–1002
Mulfort KL, Farha OK, Malliakas CD, Kanatzidis MG, Hupp JT (2010) An interpenetrated framework material with hysteretic CO2 uptake. Chem Eur J 16(1):276–281
Nakatani T, Ishimaru Y, Iida I, Furuta Y (2008) Microstructure of wood: change in micropore structure accompanied by delignification. J Wood Sci 54(3):252–255
Papadopoulos AN (2003) Determination of surface area and pore volume of holocellulose and chemically modified wood flour using the nitrogen adsorption technique. Holz Roh Werkst 61:453–456
Papadopoulos AN (2005) An investigation of the cell wall ultrastructure of the sapwood of then Greek wood species by means of chemical modification. Holz Roh Werkst 63:437–441
Quantachrome Instruments (2008) Micropore size analysis of porous carbons using CO2 adsorption at 273.15 K (0°C). Powder Tech Note 35
Quantachrome Instruments (2011) Adsorptives for physisorption experiments: selection and their physical properties. Powder Tech Note 52
Sano Y (2004) Intervascular pitting across the annual ring boundary in Betula platyphylla var. japonica and Fraxinus mandshurica var. japonica. IAWA J 25(2):129–140
Sano Y, Kawakami Y, Ohtani J (1999) Variation in the structure of intertracueary pit membranes in Abies saculinensis, as observed by field-emission scanning electron microscopy. IAWA J 20(4):375–388
Satterthwaite FE (1946) An approximate distribution of estimates of variance components. Biom Bull 2(6):110–114
Shi J, Avramidis S (2017a) Water sorption hysteresis in wood: I review and experimental patterns—geometric characteristics of scanning curves. Holzforschung 71(4):307–316
Shi J, Avramidis S (2017b) Water sorption hysteresis in wood: III physical modeling by molecular simulation. Holzforschung 71(9):733–741
Siau JF (1995) Wood: influence of moisture on physical properties. Department of Wood Science and Forest Products, Virginia Polytechnic Institute and State University, Blacksburg
Skaar C (1972) Water in wood. Syracuse University Press, New York
Stamm AJ (1929) Density of wood substance, adsorption by wood, and permeability of wood. J Phys Chem 33(3):398–414
Stamm AJ (1964) Wood and cellulose science. The Ronald Press Company, New York
Stone JE, Scallan AM (1968) The effect of component removal upon the porous structure of the cell wall of wood. Part III. A comparison between the sulphite and kraft processes. Pulp Pap Mag Can 69(6):69–74
Streit W, Fengel D (1994) Heartwood formation in Quebracho colorado (Schinopsis balansae Engl.): tannin distribution and penetration of extractives into the cell walls. Holzforschung 48(5):361–367
Swan EP, Jiang KS, Gardner JAF (1969) The lignans of Thuja plicata and the sapwood-heartwood transformation. Phytochemistry 8(2):345–351
Tarkow H, Krueger J (1961) Distribution of hot-water soluble material in cell walls and cavities of redwood. For Prod J 11(5):228–229
Vaidhyanathan R, Iremonger SS, Dawson KW, Shimizu GK (2009) An amine-functionalized metal organic framework for preferential CO2 adsorption at low pressures. Chem Commun 35:5230–5232
Welch BL (1947) The generalization of ‘student’s’ problem when several different population variances are involved. Biometrika 34(1/2):28–35
Wilfong JG (1966) Specific gravity of wood substance. For Prod J 16(1):55–61
Wilkerson CG, Mansfield SD, Lu F, Withers S, Park JY, Karlen SD, Gonzales-Vigil E, Padmakshan D, Unda F, Rencoret J, Ralph J (2014) Monolignol ferulate transferase introduces chemically labile linkages into the lignin backbone. Science 344(6179):90–93
Yang S, Lin X, Lewis W, Suyetin M, Bichoutskaia E, Parker JE, Tang CC, Allan DR, Rizkallah PJ, Hubberstey P, Champness NR (2012) A partially interpenetrated metal–organic framework for selective hysteretic sorption of carbon dioxide. Nat Mater 11(8):710–716
Yu H, Tian M, Shen C, Wang Z (2013) Facile preparation of porous polybenzimidazole networks and adsorption behavior of CO2 gas, organic and water vapors. Polym Chem 4(4):961–968
Acknowledgements
The authors thank Eric Fu from the Department of Statistics at the University of British Columbia for the statistical consulting and Dr. Katsuhiko Takata, Dr. Kayo Kudo and Dr. Yasuo Kawai from Akita Prefectural University for discussion of pit membrane structures. This work was funded by the Natural Sciences and Engineering Research Council (NSERC) Discovery Grant RGPIN-2016-04325.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, J., Avramidis, S. Dried cell wall nanopore configuration of Douglas-fir, western red cedar and aspen heartwoods. Wood Sci Technol 52, 1025–1037 (2018). https://doi.org/10.1007/s00226-018-1011-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-018-1011-4