Abstract
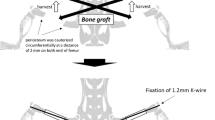
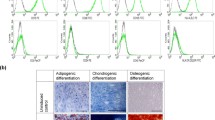
This study aimed to investigate the effects of recombinant human bone morphogenetic protein (rhBMP-7) on human cancellous bone grafts (BGs) while differentiating between anabolic and catabolic events. Human BGs alone or supplemented with rhBMP-7 were harvested 14 weeks after subcutaneous implantation into NOD/Scid mice, and studied via micro-CT, histomorphometry, immunohistochemistry and flow cytometry. Immunohistochemical staining for human-specific proteins made it possible to differentiate between grafted human bone and newly formed murine bone. Only BGs implanted with rhBMP-7 formed an ossicle containing a functional hematopoietic compartment. The total ossicle volume in the BMP+ group was higher than in the BMP− group (835 mm3 vs. 365 mm3, respectively, p < 0.001). The BMP+ group showed larger BM spaces (0.47 mm vs. 0.28 mm, p = 0.002) and lower bone volume-to-total volume ratio (31% vs. 47%, p = 0.002). Immunohistochemical staining for human-specific proteins confirmed a higher ratio of newly formed bone area (murine) to total area (0.12 vs. 0.001, p < 0.001) in the BMP+ group, while the ratio of grafted bone (human) area to total area was smaller (0.14 vs. 0.34, p = 0.004). The results demonstrate that rhBMP-7 induces BG resorption at a higher rate than new bone formation while creating a haematopoietic niche. Clinicians therefore need to consider the net catabolic effect when rhBMP-7 is used with BGs. Overall, this model indicates its promising application to further decipher BMPs action on BGs and its potential in complex bone tissue regeneration.







Similar content being viewed by others
References
Urist MR (1965) Bone: formation by autoinduction. Science 150(3698):893–899
Ripamonti U, Reddi AH (1997) Tissue engineering, morphogenesis, and regeneration of the periodontal tissues by bone morphogenetic proteins. Crit Rev Oral Biol Med 8(2):154–163
Wang EA, Rosen V, D’Alessandro JS, Bauduy M, Cordes P, Harada T et al (1990) Recombinant human bone morphogenetic protein induces bone formation. Proc Natl Acad Sci USA 87(6):2220–2224
Wozney JM, Rosen V (1998) Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res 346:26–37
Scarfi S (2016) Use of bone morphogenetic proteins in mesenchymal stem cell stimulation of cartilage and bone repair. World J Stem Cells 8(1):1–12
Mi M, Jin H, Wang B, Yukata K, Sheu TJ, Ke QH et al (2013) Chondrocyte BMP2 signaling plays an essential role in bone fracture healing. Gene 512(2):211–218
James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang X et al (2016) A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng Part B Rev 22(4):284–297
Tannoury CA, An HS (2014) Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J 14(3):552–559
Schmidmaier G, Capanna R, Wildemann B, Beque T, Lowenberg D (2009) Bone morphogenetic proteins in critical-size bone defects: what are the options? Injury 40(Suppl 3):S39–S43
Giannoudis PV, Tzioupis C (2005) Clinical applications of BMP-7: the UK perspective. Injury 36(Suppl 3):S47–S50
Aspenberg P, Linder L (2001) Impaction grafting. Acta Orthop Scand 72(2):198–199
Wang W, Yeung KWK (2017) Bone grafts and biomaterials substitutes for bone defect repair: a review. Bioact Mater 2(4):224–247
Capanna R, Campanacci DA, Caldora P, De Biase P (2005) Healing of large bone defects with allogenic bone grafts enriched with autologous bone marrow buffy coat and platelet-rich plasma. Orthop Proc 87-B(SUPP_I):59
Sen MK, Miclau T (2007) Autologous iliac crest bone graft: should it still be the gold standard for treating nonunions? Injury 38(Suppl 1):S75–S80
Ng VY (2012) Risk of disease transmission with bone allograft. Orthopedics. 35(8):679–681
Rudert M, Holzapfel BM, von Rottkay E, Holzapfel DE, Noeth U (2015) Impaction bone grafting for the reconstruction of large bone defects in revision knee arthroplasty. Oper Orthop Traumatol 27(1):35–46
Doppelt SH, Tomford WW, Lucas AD, Mankin HJ (1981) Operational and financial aspects of a hospital bone bank. J Bone Joint Surg Am 63(9):1472–1481
Jr. De Long WG, Einhorn TA, Koval K, McKee M, Smith W, Sanders R et al (2007) Bone grafts and bone graft substitutes in orthopaedic trauma surgery. A critical analysis. J Bone Joint Surg Am 89(3):649–658
Faundez A, Tournier C, Garcia M, Aunoble S, Le Huec JC (2016) Bone morphogenetic protein use in spine surgery-complications and outcomes: a systematic review. Int Orthop 40(6):1309–1319
Papanagiotou M, Dailiana ZH, Karachalios T, Varitimidis S, Vlychou M, Hantes M et al (2015) RhBMP-7 for the treatment of nonunion of fractures of long bones. Bone Joint J 97-b(7):997–1003
Granholm S, Henning P, Lindholm C, Lerner UH (2013) Osteoclast progenitor cells present in significant amounts in mouse calvarial osteoblast isolations and osteoclastogenesis increased by BMP-2. Bone. 52(1):83–92
Zheng Y, Wang L, Zhang X, Zhang X, Gu Z, Wu G (2012) BMP2/7 heterodimer can modulate all cellular events of the in vitro RANKL-mediated osteoclastogenesis, respectively, in different dose patterns. Tissue Eng Part A 18(5–6):621–630
Mathavan N, Bosemark P, Isaksson H, Tagil M (2013) Investigating the synergistic efficacy of BMP-7 and zoledronate on bone allografts using an open rat osteotomy model. Bone 56(2):440–448
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25(7):1468–1486
Holzapfel BM, Hutmacher DW, Nowlan B, Barbier V, Thibaudeau L, Theodoropoulos C et al (2015) Tissue engineered humanized bone supports human hematopoiesis in vivo. Biomaterials 61:103–114
Shahtalebi MA, Asghari GR, Rahmani F, Shafiee F, Jahanian-Najafabadi A (2018) Formulation of herbal gel of antirrhinum majus extract and evaluation of its anti-propionibacterium acne effects. Adv Biomed Res 7:53
Shafiee A, McGovern JA, Lahr CA, Meinert C, Moi D, Wagner F et al (2018) Immune system augmentation via humanization using stem/progenitor cells and bioengineering in a breast cancer model study. Int J Cancer 143:1470–1482
Thibaudeau L, Quent VM, Holzapfel BM, Taubenberger AV, Straub M, Hutmacher DW (2014) Mimicking breast cancer-induced bone metastasis in vivo: current transplantation models and advanced humanized strategies. Cancer Metastasis Rev 33(2–3):721–735
Wagner F, Holzapfel BM, Martine LC, McGovern J, Lahr CA, Boxberg M et al (2019) A humanized bone microenvironment uncovers HIF2 alpha as a latent marker for osteosarcoma. Acta Biomater 89:372–381
Hesami P, Holzapfel BM, Taubenberger A, Roudier M, Fazli L, Sieh S et al (2014) A humanized tissue-engineered in vivo model to dissect interactions between human prostate cancer cells and human bone. Clin Exp Metastasis 31(4):435–446
Holzapfel BM, Wagner F, Loessner D, Holzapfel NP, Thibaudeau L, Crawford R et al (2014) Species-specific homing mechanisms of human prostate cancer metastasis in tissue engineered bone. Biomaterials 35(13):4108–4115
Holzapfel BM, Wagner F, Thibaudeau L, Levesque JP, Hutmacher DW (2015) Concise review: humanized models of tumor immunology in the 21st century: convergence of cancer research and tissue engineering. Stem Cells 33(6):1696–1704
Hutmacher DW, Holzapfel BM, De-Juan-Pardo EM, Pereira BA, Ellem SJ, Loessner D et al (2015) Convergence of regenerative medicine and synthetic biology to develop standardized and validated models of human diseases with clinical relevance. Curr Opin Biotechnol 35:127–132
Thibaudeau L, Holzapfel BM, Hutmacher DW (2015) Humanized mice models for primary bone tumor and bone metastasis research. Cell Cycle 14(14):2191–2192
Seib FP, Berry JE, Shiozawa Y, Taichman RS, Kaplan DL (2015) Tissue engineering a surrogate niche for metastatic cancer cells. Biomaterials 51:313–319
Zhang J, Niu C, Ye L, Huang H, He X, Tong WG et al (2003) Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425(6960):836–841
Su YH, Cai HB, Ye ZY, Tan WS (2015) BMP-7 improved proliferation and hematopoietic reconstitution potential of ex vivo expanded cord blood-derived CD34(+) cells. Hum Cell 28(1):14–21
Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I et al (2007) Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 131(2):324–336
Larsson J, Karlsson S (2005) The role of Smad signaling in hematopoiesis. Oncogene 24(37):5676–5692
Wagner F, Holzapfel BM, McGovern JA, Shafiee A, Baldwin JG, Martine LC et al (2018) Humanization of bone and bone marrow in an orthotopic site reveals new potential therapeutic targets in osteosarcoma. Biomaterials 171:230–246
Belfrage O, Flivik G, Sundberg M, Kesteris U, Tagil M (2011) Local treatment of cancellous bone grafts with BMP-7 and zoledronate increases both the bone formation rate and bone density: a bone chamber study in rats. Acta Orthop 82(2):228–233
Bougioukli S, Jain A, Sugiyama O, Tinsley BA, Tang AH, Tan MH et al (2016) Combination therapy with BMP-2 and a systemic RANKL inhibitor enhances bone healing in a mouse critical-sized femoral defect. Bone 84:93–103
Karrholm J, Hourigan P, Timperley J, Razaznejad R (2006) Mixing bone graft with OP-1 does not improve cup or stem fixation in revision surgery of the hip: 5-year follow-up of 10 acetabular and 11 femoral study cases and 40 control cases. Acta Orthop 77(1):39–48
Yoshioka Y, Ono M, Osaki M, Konishi I, Sakaguchi S (2012) Differential effects of inhibition of bone morphogenic protein (BMP) signalling on T-cell activation and differentiation. Eur J Immunol 42(3):749–759
Rocher C, Singla R, Singal PK, Parthasarathy S, Singla DK (2012) Bone morphogenetic protein 7 polarizes THP-1 cells into M2 macrophages. Can J Physiol Pharmacol 90(7):947–951
Singla DK, Singla R, Wang J (2016) BMP-7 treatment increases M2 macrophage differentiation and reduces inflammation and plaque formation in Apo E−/− mice. PLoS ONE 11(1):e0147897
Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T et al (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93(2):165–176
Kamiya N, Mishina Y (2011) New insights on the roles of BMP signaling in bone-A review of recent mouse genetic studies. BioFactors (Oxford, England). 37(2):75–82
Wutzl A, Brozek W, Lernbass I, Rauner M, Hofbauer G, Schopper C et al (2006) Bone morphogenetic proteins 5 and 6 stimulate osteoclast generation. J Biomed Mater Res A 77(1):75–83
Zhu W, Kim J, Cheng C, Rawlins BA, Boachie-Adjei O, Crystal RG et al (2006) Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone 39(1):61–71
Acknowledgements
Baxter Healthcare Australia kindly provided Fibrin Glue (TISSEEL Fibrin Sealant). The following funding existed during conduction of this study: National Health and Medical Research Council of Australia (Project Grant 1082313 to BMH and DWH), German Research Foundation (DFG HO 5068/1-1 to BMH) and Australian Research Council (IC160100026).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Christoph A. Lahr, Ferdinand Wagner, Abbas Shafiee, Maximilian Rudert, Dietmar W. Hutmacher and Boris Michael Holzapfel declare that there is no conflict of interest.
Human and Animal Rights and Informed Consent
This study was approved by the University Animal Ethics Committee (Approval No. 130/025). All animal experiments were executed in conformity with the national animal care guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lahr, C.A., Wagner, F., Shafiee, A. et al. Recombinant Human Bone Morphogenetic Protein 7 Exerts Osteo-Catabolic Effects on Bone Grafts That Outweigh Its Osteo-Anabolic Capacity. Calcif Tissue Int 105, 331–340 (2019). https://doi.org/10.1007/s00223-019-00574-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00574-5